Glycerol catches fire on mixing with:
1.
2.
3.
4. None of the above
The following transformation involves a carbocation rearrangement. The carbocation is generated by protonation of the hydroxyl group, followed by the by the loss of water. Which bond has to imagine in the carbocation to yield the product indicated (after the deprotonation) ?
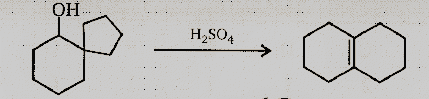
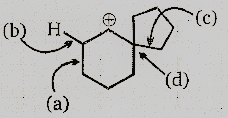
(1) a
(2) b
(3) c
(4) d
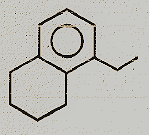
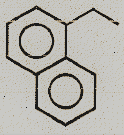
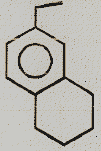
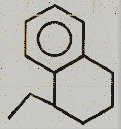
Identify the major product.
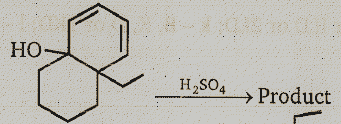
(1) 
(2) 
(3) 
(4) 
The compound among the following that is most easily oxidised is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
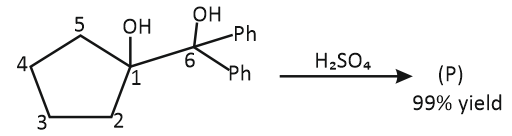 ;
;
Unknown (P) of the reaction is:
| 1. | 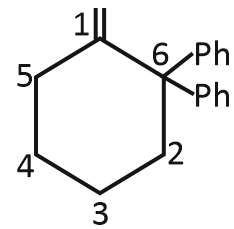 |
2. | 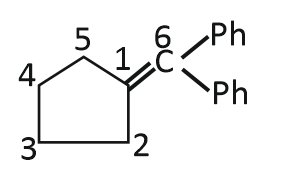 |
| 3. | 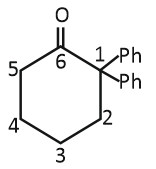 |
4. | 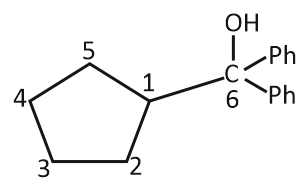 |
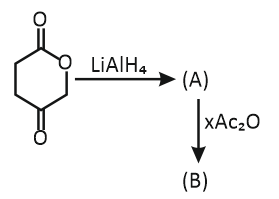
In the above reaction, X indicates the moles of anhydride consumed.
How many moles of X are required for the reaction of (A) with
acetic anhydride to form product (B)?
1. One (1)
2. Two (2)
3. Three (3)
4. Four (4)
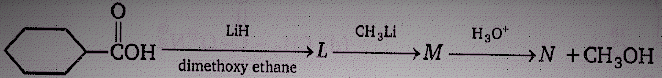
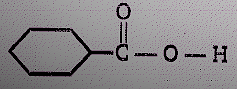
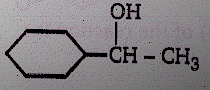
Product (N) is :
(1) 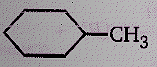
(2) 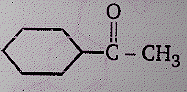
(3) 
(4) 
Which of the following statements is true?
1. CH3CH2S- is both a stronger base and more nucleophilic than CH3CH2O-
2. CH3CH2S- is a stronger base but is less nucleophilic than CH3CH2O-
3. CH3CH2S- is a weaker base but is more nucleophilic than CH3CH2O-
4. CH3CH2S- is both a weaker base and less nucleophilic than CH3CH2O-
Identify the product (P) in the given reaction:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Which of the following react with HBr at faster rate?








