When NH4Cl is added in NH4OH solution, then pH of the solution
1. Increases
2. Decreases
3. Remains unchanged
4. Firstly decreases and more than decreases
The most hydrolyzed salt among the following is-
(Assume that Kb of all weak bases is the same)
1. NH4Cl
2. CuSO4
3. AlCl3
4. All are equally hydrolyzed.
The pH of 10-6 M CH3COOH (Ka = 1.8 X 10-5) is:
1. 5.37
2. In between 6 & 7
3. 7
4. 8.63
60 ml 1M CH3COOH is mixed with 20 ml 1 M NaOH then pH of resulting solution will be
(Ka = 1.8 X 10-5, log1.8 = 0.25)
1. 8.55
2. 4.95
3. 4.45
4. 7.45
In which solvent, solubility of AgCl is maximum ?
1. 0.01 M NaCl
2. Pure Water
3. 0.01 M NH4OH
4. 0.05 M AgNO3
for the gaseous reaction:
Would be respectively:
1. (RT)-3, (RT)2, (RT)0
2. (RT)-3, (RT)-2, (RT)-1
3. (RT)-3, (RT)2, (RT)
4. None of the above
N2O4 dissociates as at 273 K and 2 atm pressure. The equilibrium mixture has a vapour density of 41. What will be the percentage degree of dissociation ?
1. 14.2%
2. 16.2%
3. 12.2%
4. 87.8%
Inert gas is added to the equilibrium at constant pressure. The degree of dissociation will :
1. Remain unchanged
2. Decrease
3. Increase
4. Decrease or increase but cannot be predicted with certainty
Dissociation of phosphorus pentachloride is favoured by:
1. High temperature and high pressure
2. High temperature and low pressure
3. Low temperature and low pressure
4. Low temperature and high pressure
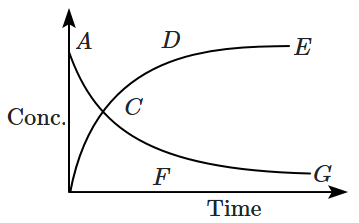
| I. | The reaction quotient (Q) has a maximum value at point A. |
| II. | The reaction proceeds left to right at a point when \(\left[\mathrm{N}_2 \mathrm{O}_4\right]=\left[\mathrm{NO}_2\right]=0.1 \mathrm{M}\) |
| III. | Kc=Q when point E or G is reached. |
1. I, and II
2. II, and III
3. I, and III
4. I,II ,and III






