N2O4 dissociates as at 273 K and 2 atm pressure. The equilibrium mixture has a vapour density of 41. What will be the percentage degree of dissociation ?
1. 14.2%
2. 16.2%
3. 12.2%
4. 87.8%
Inert gas is added to the equilibrium at constant pressure. The degree of dissociation will :
1. Remain unchanged
2. Decrease
3. Increase
4. Decrease or increase but cannot be predicted with certainty
Dissociation of phosphorus pentachloride is favoured by:
1. High temperature and high pressure
2. High temperature and low pressure
3. Low temperature and low pressure
4. Low temperature and high pressure
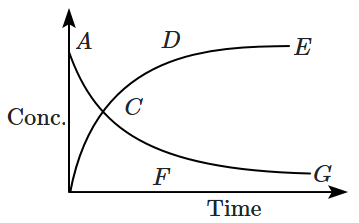
| I. | The reaction quotient (Q) has a maximum value at point A. |
| II. | The reaction proceeds left to right at a point when \(\left[\mathrm{N}_2 \mathrm{O}_4\right]=\left[\mathrm{NO}_2\right]=0.1 \mathrm{M}\) |
| III. | Kc=Q when point E or G is reached. |
1. I, and II
2. II, and III
3. I, and III
4. I,II ,and III
The stability product constant Ksp of Mg(OH)2 is 9.0 X 10-12. If a solution is 0.010 M with respect to Mg2+ ion, what is the maximum hydroxide ion concentration which could be present without causing the precipitation of Mg(OH)2 ?
1. 1.5 X 10-7 M
2. 3.0 X 10-7 M
3. 1.5 X 10-5 M
4. 3.0 X 10-5 M
The amount of CaC2O4 (molecular weight =128) will dissolve in distilled water to make 1 liter of a saturated solution is:
[Ksp (CaC2O4) = 2.5 x 10-9 ]
1. 0.0064 g
2. 0.1280 g
3. 0.0128 g
4. 1.2800 g
What substance could be added to one liter of water to serve as a buffer?
1. One mole of \(CH_
3
COO
H\) and 0.5 moles of \(NaOH\)
2. One mole of \(NH_4Cl\) and one mole of \(HCl\)
3. One mole of \(NH_4OH\) and one mole of \(NaOH\)
4. One mole of \(CH_ 3 COO H\) and one mole of \(HCl\)
Acoording to Bronsted-Lowry concept, the correct order of relative strength of bases follows the order
1. CH3COO- > Cl- > OH-
2. CH3COO- > OH- > Cl-
3. OH- > CH3COO- > Cl-
4. OH- > Cl-> CH3COO-
pOH of H2O is 7.0 at 298K. If water is heated at 350K, which of the following statement should be true ?
(1) pOH will decrease
(2) pOH will increase
(3) pOH will remain 7.0
(4) Concentration of H+ ions will increase but that of OH- will decrease.
An aqueous solution of NaHSO3 is-
1. Acidic
2. Slightly alkaline
3. Alkaline
4. Slightly Acidic






