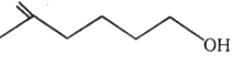
 W. Product W is:
W. Product W is:
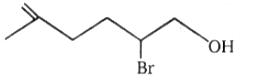
1. 

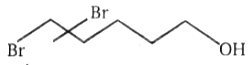
2. 

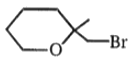
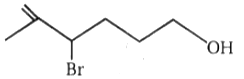
3. 

4. 


 W. Product W is:
W. Product W is:







In each of the following groups, which is the strongest (best) nucleophile ?
(I)(1)H3C-O- (2) 
(II)(1)OH- (2) H2O (3) in DMF
(III) (1) 

(a) I,3; II,3; III,2 (b) I,2; II,1; III,3
(c) I,1; II,2; III,1 (d) I,3; II,1; III,3
(A) on heating isomerizes to (B). What is the structure of (B) ?
Which of the following statements is true?
1. CH3CH2S- is both a stronger base and more nucleophilic than CH3CH2O-
2. CH3CH2S- is a stronger base but is less nucleophilic than CH3CH2O-
3. CH3CH2S- is a weaker base but is more nucleophilic than CH3CH2O-
4. CH3CH2S- is both a weaker base and less nucleophilic than CH3CH2O-
The labeled -O18 will be:
1. H2O
2. Methyl benzoate
3. Both 1 and 2
4. Benzoic acid
Product (A) in the below mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Which of the following compounds is most prone to oxidation?
| 1. | \(\mathrm{CH}_3-\mathrm{CHOH}-\mathrm{CH}_3\) |
2. |  |
| 3. | \(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{O}-\mathrm{CH}_2-\mathrm{CH}_3\) |
4. | 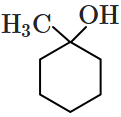 |
Which of the following react with HBr at faster rate?
| 1. | \(\mathrm{NaBH}_4 \) | 2. | \(\mathrm{LiAlH}_4 \) |
| 3. | \(PCC \) | 4. | \(\mathrm{KMnO}_4\) |

| 1. |  |
2. |  |
| 3. |  |
4. |  |












