and are converted to monocations and respectively. Which of the following is incorrect?
1. In , the bond weakens.
2. In , the bond order increases.
3. In , the paramagnetism decreases.
4. becomes diamagnetic.
The species, having bond angles of 120° is :
1. PH3
2. ClF3
3. NCl3
4. BCl3
Which of the following pairs of compounds is isoelectronic and isostructural?
(1) BeCl2, XeF2
(2) Tel2, XeF2
(3) , XeF2
(4) IF3, XeF2
Which one of the following pairs of species have the same bond order?
(1) CO, NO
(2) O2- , CN-
(3) CN- , NO+
(4) N+ , CN+
In which of the following compounds is an intramolecular hydrogen bond present?
| 1. | H2O2 | 2. | HCN |
| 3. | Cellulose | 4. | Concentrated acetic acid |
The hybridisations of atomic orbitals of nitrogen in and are
(a) sp, sp3 and sp2 (b) sp2, sp3 and sp
(c) sp, sp2 and sp3 (d) sp2, sp and sp3
The correct shape and hybridisation for XeF4 are
(1) octahedral, sp3d2
(2) trigonal bipyramidal, sp3d2
(3) planar triangle, sp3d3
(4) square planar, sp3d2
In which of the following molecules, all atoms are coplanar ?
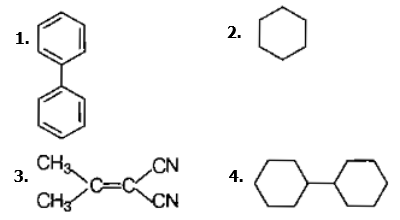
Predict the correct order among the following.
(1) lone pair-lone pair>bond pair-bond pair>lone pair-bond pair
(2) bond pair-bond pair>lone pair-bond pair>lone pair-lone pair
(3) lone pair-bond pair>bond pair-bond pair>lone pair-lone pair
(4) lone pair-lone pair>lone pair-bond pair>bond pair-bond pair
The pair of electrons in the given carbanion, CH3C≡C-, is present in which of the following orbitals?
| 1. | sp3 | 2. | sp2 |
| 3. | sp | 4. | 2p |






