Which of the following pairs of compounds is isoelectronic and isostructural?
(1) BeCl2, XeF2
(2) Tel2, XeF2
(3) , XeF2
(4) IF3, XeF2
(1) BeCl2, XeF2
(2) Tel2, XeF2
(3) , XeF2
(4) IF3, XeF2
Which one of the following pairs of species have the same bond order?
(1) CO, NO
(2) O2- , CN-
(3) CN- , NO+
(4) N+ , CN+
In which of the following compounds is an intramolecular hydrogen bond present?
| 1. | H2O2 | 2. | HCN |
| 3. | Cellulose | 4. | Concentrated acetic acid |
The hybridisations of atomic orbitals of nitrogen in and are
(a) sp, sp3 and sp2 (b) sp2, sp3 and sp
(c) sp, sp2 and sp3 (d) sp2, sp and sp3
The correct shape and hybridisation for XeF4 are
(1) octahedral, sp3d2
(2) trigonal bipyramidal, sp3d2
(3) planar triangle, sp3d3
(4) square planar, sp3d2
In which of the following molecules, all atoms are coplanar ?
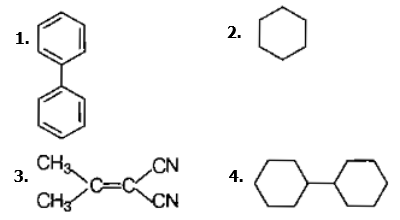
Predict the correct order among the following.
(1) lone pair-lone pair>bond pair-bond pair>lone pair-bond pair
(2) bond pair-bond pair>lone pair-bond pair>lone pair-lone pair
(3) lone pair-bond pair>bond pair-bond pair>lone pair-lone pair
(4) lone pair-lone pair>lone pair-bond pair>bond pair-bond pair
The pair of electrons in the given carbanion, CH3C≡C-, is present in which of the following orbitals?
| 1. | sp3 | 2. | sp2 |
| 3. | sp | 4. | 2p |
The incorrect statement among the following regarding the molecules of CH4, NH3, and H2O is:
| 1. | The H-O-H bond angle in H2O is larger than the H-C-H bond angle in CH4. |
| 2. | The H-O-H bond angle in H2O is smaller than the H-N-H bond angle in NH3. |
| 3. | The H-C-H bond angle in CH4 is larger than the H-N-H bond angle in NH3. |
| 4. | The H-C-H bond angle in CH4, the H-N-H bond angle in NH3, and the H-O-H bond angle in H2O are all greater than 90°. |
Which one of the following orders is correct for the bond dissociation enthalpy of halogen molecules?
1. Cl2 > Br2 > F2 > I2
2. Br2 > I2 > F2 > Cl2
3. F2 > Cl2 > Br2 > I2
4. I2 > Br2 > Cl2 > F2






