Classification of polymers on the basis of structure include-
1. Linear polymers
2. Branched-chain polymers
3. Cross-linked or Network polymers
4. All of these
The names of monomers of the following polymers are respectively -
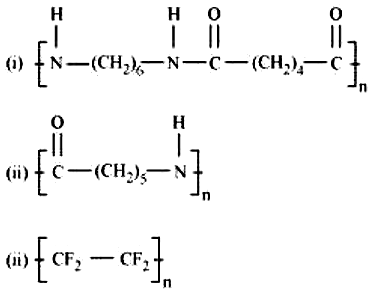
1) Hexaethylenediamine, Caprolactum, Tetrafluoroethene
2) Hexamethylenediamine, Caprolactum, Tetrafluoroethyne
3) Hexamethylenediamine, Caprolactum, Tetrafluoroethene
4) Hexaethylenediamine, Caprolactum, Tetrafluoroethane
Classify the polymers into addition and condensation polymers: Terylene, Bakelite, Polyvinyl chloride, Polythene.
| 1. | Addition polymers: Terylene, bakelite; Condensation polymers: Polyvinyl chloride, polythene |
| 2. | Addition polymers: bakelite; Condensation polymers: Terylene, bakelite, Polyvinylchloride, polythene |
| 3. | Addition polymers: polythene; Condensation polymers: Terylene, bakelite, Polyvinylchloride |
| 4. | Addition polymers: Polyvinyl chloride, polythene; Condensation polymers: Terylene, bakelite |
The difference between Buna-N and Buna-S is :
| 1. | Buna-N is a copolymer of 3−butyne, and acrylonitrile while Buna-S is a copolymer of 3−butyne, and styrene. |
| 2. | Buna-S is a copolymer of 3−butyne, and acrylonitrile while Buna-N is a copolymer of 3−butyne, and styrene. |
| 3. | Buna-N is a copolymer of 1,3−butadiene, and acrylonitrile while Buna-S is a copolymer of 1, 3−butadiene, and styrene. |
| 4. | Buna-S is a copolymer of 1,3−butadiene, and acrylonitrile while Buna-N is a copolymer of 1, 3−butadiene, and styrene. |
On the basis of molecular forces, polymer can be classified as -
1. Elastomers
2. Fibres
3. Thermoplastic polymers
4. All of the above
The term copolymerization means formation of polymer from :
1. The same monomer.
2. Two or more different monomers.
3. Acid and base.
4. Elastomer.
The examples of thermoplastics and thermosetting polymers are respectively :
| 1. | Thermoplastics:polythene, urea-formaldehyde resins ; Thermosetting : bakelite, polystyrene |
| 2. | Thermoplastics: bakelite, polystyrene; Thermosetting : polythene, urea-formaldehyde res |
| 3. | Thermoplastics:polythene, polystyrene ; Thermosetting : bakelite, urea-formaldehyde resins |
| 4. | Thermoplastics:bakelite, urea-formaldehyde resins ; Thermosetting : polythene, polystyrene |
The monomers used for getting the following polymers are respectively -
(i) Teflon (ii) Bakelite
1. i = Formaldehyde (HCHO) and phenol; ii = Vinyl chloride
2. i = Tetrafluoroethylene; and ii = Vinyl chloride
3. i = Vinyl chloride; ii = Formaldehyde (HCHO), and phenol
4. i = Tetrafluoroethylene; ii = Formaldehyde (HCHO) and phenol
One of the common initiators used in free radical addition polymerization is -
1. Toluene
2. Ethanol
3. Benzoyl peroxide
4. Acetaldehyde
The presence of double bonds in rubber molecules increase -
1. Viscosity
2. Elasticity
3. Surface Tension
4. Vapour pressure






