On the basis of molecular forces, polymer can be classified as -
1. Elastomers
2. Fibres
3. Thermoplastic polymers
4. All of the above
The term copolymerization means formation of polymer from :
1. The same monomer.
2. Two or more different monomers.
3. Acid and base.
4. Elastomer.
The examples of thermoplastics and thermosetting polymers are respectively :
| 1. | Thermoplastics:polythene, urea-formaldehyde resins ; Thermosetting : bakelite, polystyrene |
| 2. | Thermoplastics: bakelite, polystyrene; Thermosetting : polythene, urea-formaldehyde res |
| 3. | Thermoplastics:polythene, polystyrene ; Thermosetting : bakelite, urea-formaldehyde resins |
| 4. | Thermoplastics:bakelite, urea-formaldehyde resins ; Thermosetting : polythene, polystyrene |
The monomers used for getting the following polymers are respectively -
(i) Teflon (ii) Bakelite
1. i = Formaldehyde (HCHO) and phenol; ii = Vinyl chloride
2. i = Tetrafluoroethylene; and ii = Vinyl chloride
3. i = Vinyl chloride; ii = Formaldehyde (HCHO), and phenol
4. i = Tetrafluoroethylene; ii = Formaldehyde (HCHO) and phenol
One of the common initiators used in free radical addition polymerization is -
1. Toluene
2. Ethanol
3. Benzoyl peroxide
4. Acetaldehyde
The presence of double bonds in rubber molecules increase -
1. Viscosity
2. Elasticity
3. Surface Tension
4. Vapour pressure
The purpose of vulcanization of rubber is/are -
1. To maintain the elasticity of natural rubber.
2. To improve the tensile strength of natural rubber.
3. To use for a wide range of temperatures.
4. All of the above.
The monomeric repeating units of Nylon-6 and Nylon-6, 6 are respectively -
1. Nylon 6 - Caprolactam; Nylon-6,6 - hexamethylene diamine and adipic acid
2. Nylon 6 - hexamethylene diamine and adipic acid; Nylon-6,6 - Caprolactam
3. Nylon 6 - Caprolactam and adipic acid; Nylon-6,6 - hexamethylene diamine
4. Nylon 6 - pentaethylene diamine and adipic acid; Nylon-6,6 - Caprolactam
The names of the monomer/s of the following polymers are respectively -
(i) Buna-S (ii) Buna-N
(iii) Dacron (iv) Neoprene
1) 1,3-butadiene and styrene, 1,3-butadiene and acrylonitrile, terepthalic acid and ethylene glycol, chloroprene
2) 1,3-butadiene and acrylonitrile, 1,3-butadiene and styrene, chloroprene, terepthalic acid and ethylene glycol
3) 1,3-butadiene and styrene, 1,3-butadiene and acrylonitrile, chloroprene, terepthalic acid and ethylene glycol
4) Chloroprene, terepthalic acid and ethylene glycol, 1,3-butadiene and styrene, 1,3-butadiene and acrylonitrile
The monomer in the following polymeric structures are respectively -
(i)
(ii)
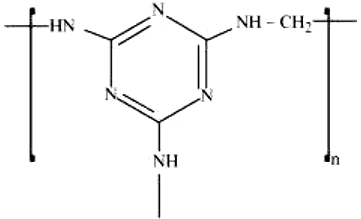
1. Decanoic acid and hexamethylene diamine; melamine and formaldehyde
2. Hexamethylene diamine and formaldehyde; hexamethylene diamine and decanoic acid
3. Decanoic acid and tetramethylene diamine; tetramethylene diamine and formaldehyde
4. Tetramethylene diamine and formaldehyde; decanoic acid and tetramethylene diamine







