Maxwell's speed distribution graph is drawn as shown below. The most probable speed of the gas molecules is:

1. 4 km/s
2. Between 3 km/s and 1 km/s
3. Any value between 2 km/s and 6 km/s
4. More than 4 km/s

1. 4 km/s
A closed container having an ideal gas is heated gradually to increase the temperature by 20% The mean free path will become/remain:
1. 20% more
2. Same
3. 20% less
4. 33% less
| 1. | \(3:1\) | 2. | \(9:1\) |
| 3. | \(1:1\) | 4. | \(1:4\) |
When the gas in an open container is heated, the mean free path:
1. Increases
2. Decreases
3. Remains the same
4. Any of the above depending on the molar mass
From the following V-T diagram, we can conclude
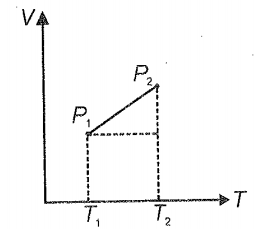
1. =
2. >
3. <
4.
Sorry!! currently, the explanation for the question is not provided. If you need further help, please email at support@neetprep.com with subject: Explanation Missing for Question Id: 160063
How does the temperature change when the state of an ideal gas is changed according to the process shown in the figure?
| 1. | temperature increases continuously. |
| 2. | temperature decreases continuously. |
| 3. | temperature first increases and then decreases. |
| 4. | temperature first decreases and then increases. |
What is the ratio of temperatures
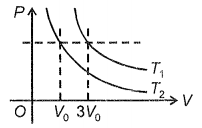
1.
2. 3 : 1
3. 9 : 1
4. 27 : 1
The mean free path for a gas, with molecular diameter \(d\) and number density \(n,\) can be expressed as:
| 1. | \( \dfrac{1}{\sqrt{2} n \pi {d}^2} \) | 2. | \( \dfrac{1}{\sqrt{2} n^2 \pi {d}^2} \) |
| 3. | \(\dfrac{1}{\sqrt{2} n^2 \pi^2 d^2} \) | 4. | \( \dfrac{1}{\sqrt{2} n \pi {d}}\) |
A cylinder contains hydrogen gas at a pressure of \(249~\text{kPa}\) and temperature \(27^\circ\text{C}.\) Its density is:
(\(R=8.3~\text{J mol}^{-1} \text {K}^{-1}\))
1. \(0.2~\text{kg/m}^{3}\)
2. \(0.1~\text{kg/m}^{3}\)
3. \(0.02~\text{kg/m}^{3}\)
4. \(0.5~\text{kg/m}^{3}\)
(\(k_B\) is Boltzmann constant and \(T\) absolute temperature)
| 1. | \(\dfrac{3}{2}k_BT\) | 2. | \(\dfrac{5}{2}k_BT\) |
| 3. | \(\dfrac{7}{2}k_BT\) | 4. | \(\dfrac{1}{2}k_BT\) |







