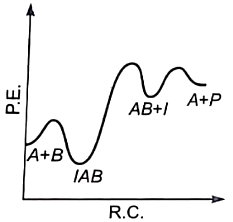
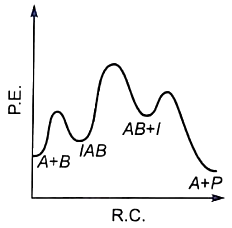
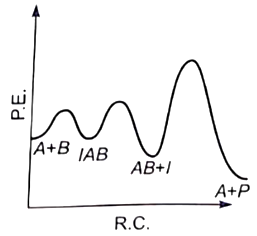
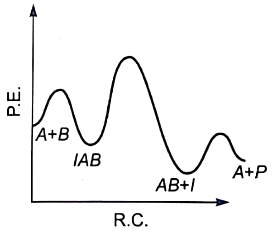
The plot of log k vs helps to calculate :
1. Energy of activation.
2. Rate constant of reaction.
3. Order of the reaction.
4. Energy of activation as well as the frequency factor.
The effect of a catalyst in a chemical reaction is
to change the
1. Acivation energy
2. Equilibrium concentration
3. Heat of reaction
4. Final products
A hydrogenation reaction is carried out at 500 K. If the same reaction is carried out in presence of a catalyst at the same rate with same frequency factor, the temperature required is 400 K the activation energy of the reaction, if the catalyst lowers the activation energy barrier by 16 kJ/mol is:
1. 100 kJ/mol
2. 80 kJ/mol
3. 60 kJ/mol
4. None of th above
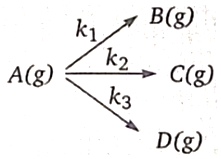
1. 0.288
2. 0.577
3. 1.154
4. None of these
The gaseous decomposition reaction, A(g) 2B(g) + C(g) is observed to first order over the excess of liquid water at It is found that after 10 minutes the total pressure of the system is 188 torr and after a very long time, it is 388 torr. The rate constant of the reaction is :
[Given : vapour pressure of at is 28 torr (In 2 = 0.7, In 3 = 1.1, In 10 = 2.3)]
1. 0.02
2. 1.2
3. 0.2
4. None of these
1. \(3 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1} \)
2. \(4 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1} \)
3. \(6 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1} \)
4. \(1 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)