Maxwell's velocity distribution curve is given for two different temperatures. For the given curves-
1.
2.
3.
4.
Maxwell's speed distribution graph is drawn as shown below. The most probable speed of the gas molecules is:
1. 4 km/s
2. Between 3 km/s and 1 km/s
3. Any value between 2 km/s and 6 km/s
4. More than 4 km/s
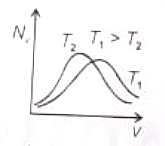
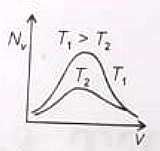
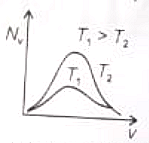
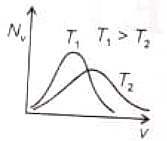
The effect of temperature on Maxwell's speed distribution is correctly shown by:
(1) 
(2) 
(3) 
(4) 
Select the incorrect statement about Maxwell's speed distribution curve.
(1) The distribution function depends only on the absolute temperature.
(2)
(3) The area under the distribution curve gives the total number of molecules of the gas.
(4) The distribution curve is symmetric about the most probable speed.
A vessel of volume V contains a mixture of 1 mole of hydrogen and 1 mole of oxygen (both considered ideal). Let denotes the fraction of molecules with a speed between v and (v+dv) with (v)dv similarly for oxygen. Then,
1. (v) = f(v) obeys the Maxwell's distribution law.
2. will obey Maxwell’s distribution law separately.
3. neither nor will obey Maxwell’s distribution law.
4. and will be the same.




