Temperature is defined by
1. First Law of thermodynamics
2. Second Law of thermodynamics
3. Third Law of thermodynamics
4. Zeroth Law of thermodynamics
Which of the following is not thermodynamical function ?
(1) Enthalpy
(2) Work done
(3) Gibb's energy
(4) Internal energy
Which of the following can not determine the state of a thermodynamic system?
| 1. | pressure and volume |
| 2. | volume and temperature |
| 3. | temperature and pressure |
| 4. | any one of pressure, volume, or temperature |
Which of the following is not a thermodynamics co-ordinate ?
(1) P
(2) T
(3) V
(4) R
In an isothermal change, an ideal gas obeys:
| 1. | Boyle's law | 2. | Charles law |
| 3. | Gay-Lussac law | 4. | None of the above |
A system goes from A to B via two processes I and II as shown in figure. If ΔUI and ΔUII are the changes in internal energies in the processes I and II respectively, then
(1) ΔUII > ΔUI
(2) ΔUII < ΔUI
(3) ΔUI = ΔUII
(4) Relation between ΔUI and ΔUII can not be determined
In the given (V-T) diagram, what is the relation between pressures ?
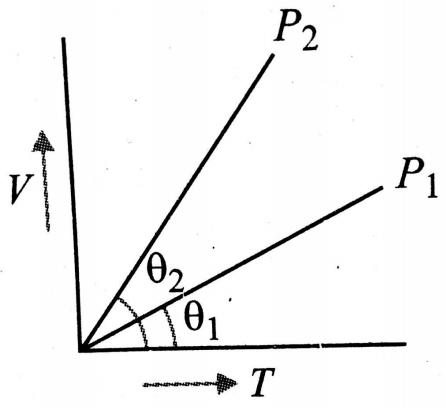
1.
2.
3. Cannot be predicted
4.
Given below are two statements:
| Statement I: | Zeroth law of thermodynamics explains the concept of thermal energy. |
| Statement II: | Thermal energy is independent of temperature. |
| 1. | Statement I is False but Statement II is True. |
| 2. | Both Statement I and Statement II are True. |
| 3. | Both Statement I and Statement II are False. |
| 4. | Statement I is True but Statement II is False. |
In thermodynamics, the Zeroth law is related to:
1. Work done
2. Thermal equilibrium
3. Entropy
4. Diffusion







