In the given (V-T) diagram, what is the relation between pressures ?
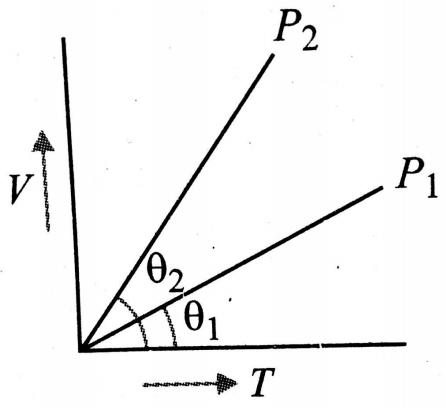
1.
2.
3. Cannot be predicted
4.

When of ice at melts to water at , the resulting change in its entropy, taking latent heat of ice to be is
1.
2.
3.
4.
Thermodynamic processes are indicated in the following diagram:
Match the following:
| Column-I | Column-II | ||
| (P) | Process I | (a) | Adiabatic |
| (Q) | Process II | (b) | Isobaric |
| (R) | Process III | (c) | Isochoric |
| (S) | Process IV | (d) | Isothermal |
| 1. | P → c, Q → a, R → d, S→ b |
| 2. | P→ c, Q → d, R → b, S → a |
| 3. | P → d, Q → b, R → b, S → c |
| 4. | P → a, Q → c, R → d, S → b |
A sample of \(0.1\) g of water at \(100^{\circ}\mathrm{C}\) and normal pressure (\(1.013 \times10^5\) N m–2) requires \(54\) cal of heat energy to convert it into steam at \(100^{\circ}\mathrm{C}\). If the volume of the steam produced is \(167.1\) cc, then the change in internal energy of the sample will be:
| 1. | \(104.3\) J | 2. | \(208.7\) J |
| 3. | \(42.2\) J | 4. | \(84.5\) J |
| 1. | \(\dfrac{3}{2}R\) | 2. | \(\dfrac{5}{2}R\) |
| 3. | \(2R\) | 4. | \(R\) |
The volume \((V)\) of a monatomic gas varies with its temperature \((T),\) as shown in the graph. The ratio of work done by the gas to the heat absorbed by it when it undergoes a change from state \(A\) to state \(B\) will be:

| 1. | \(\dfrac{2}{5}\) | 2. | \(\dfrac{2}{3}\) |
| 3. | \(\dfrac{1}{3}\) | 4. | \(\dfrac{2}{7}\) |
| 1. | \(26.8\%\) | 2. | \(20\%\) |
| 3. | \(6.25\%\) | 4. | \(12.5\%\) |
| Assertion (A): | Thermodynamic process in nature are irreversible. |
| Reason (R): | Dissipative effects cannot be eliminated. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
(Take, 1 cal= 4.2 Joules)
1. 23.65 W
2. 236.5 W
3. 2365 W
4. 2.365 W
An ideal gas is compressed to half its initial volume using several processes. Which of the processes results in the maximum work done on the gas?
1. adiabatic
2. isobaric
3. isochoric
4. isothermal







