An ideal heat engine working between temperatures T1 and T2 has an efficiency η. The new efficiency if both the source and sink temperatures are doubled will be:
1.
2. η
3. 2η
4. 3η
1.
2. η
3. 2η
4. 3η
A monoatomic ideal gas, initially at temperature \(T_1\), is enclosed in a cylinder fitted with a frictionless piston. The gas is allowed to expand adiabatically to a temperature \(T_2\) by releasing the piston suddenly. If \(L_1\) and \(L_2\) are the lengths of the gas column before and after expansion, respectively, then \(\frac{T_1}{T_2}\) is given by:
1. \(\left(\frac{L_1}{L_2}\right)^{\frac{2}{3}}\)
2. \(\frac{L_1}{L_2}\)
3. \(\frac{L_2}{L_1}\)
4. \(\left(\frac{L_2}{L_1}\right)^{\frac{2}{3}}\)
The initial pressure and volume of a gas are \(P\) and \(V\), respectively. First, it is expanded isothermally to volume \(4V\) and then compressed adiabatically to volume \(V\). The final pressure of the gas will be: [Given: \(\gamma = 1.5\)]
| 1. | \(P\) | 2. | \(2P\) |
| 3. | \(4P\) | 4. | \(8P\) |
If n moles of an ideal gas is heated at a constant pressure from 50°C to 100°C, the increase in the internal energy of the gas will be: \(\left(\frac{C_{p}}{C_{v}} = \gamma\ and\ R = gas\ constant\right)\)
| 1. | \(\frac{50 nR}{\gamma - 1}\) | 2. | \(\frac{100 nR}{\gamma - 1}\) |
| 3. | \(\frac{50 nγR}{\gamma - 1}\) | 4. | \(\frac{25 nγR}{\gamma - 1}\) |
The latent heat of vaporisation of water is \(2240~\text{J/gm}\). If the work done in the process of expansion of \(1~\text{g}\) is \(168~\text{J}\),
then the increase in internal energy is:
1. \(2408~\text{J}\)
2. \(2240~\text{J}\)
3. \(2072~\text{J}\)
4. \(1904~\text{J}\)
The figure below shows two paths that may be taken by a gas to go from state A to state C. In process AB, \(400~\text{J}\) of heat is added to the system and in process BC, \(100~\text{J}\) of heat is added to the system. The heat absorbed by the system in the process AC will be:
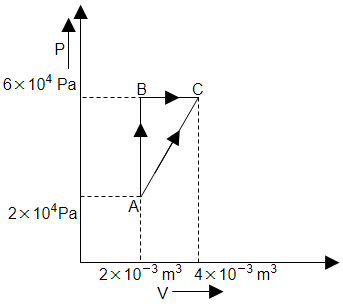
| 1. | \(380~\text{J}\) | 2. | \(500~\text{J}\) |
| 3. | \(460~\text{J}\) | 4. | \(300~\text{J}\) |
The first law of thermodynamics is based on:
| 1. | the concept of temperature. |
| 2. | the concept of conservation of energy. |
| 3. | the concept of working of heat engine. |
| 4. | the concept of entropy. |
The efficiency of an ideal heat engine is less than 100% because of:
| 1. | the presence of friction. |
| 2. | the leakage of heat energy. |
| 3. | unavailability of the sink at zero kelvin. |
| 4. | All of these |
Work done during the given cycle is:
1. 4
2. 2
3.
4.
An ideal gas goes from A to B via two processes, l and ll, as shown. If and are the changes in internal energies in processes I and II, respectively, then (\(P:\) pressure, \(V:\) volume)
| 1. | ∆U1 > ∆U2 | 2. | ∆U1 < ∆U2 |
| 3. | ∆U1 = ∆U2 | 4. | ∆U1 ≤ ∆U2 |




