An organic compound A on reduction gives compound B which on reaction with chloroform and potassium hydroxide forms C. The compound C on catalytic reduction gives N-methylaniline. The compound A is [2000]
1. nitrobenzene
2. nitromethane
3. methylamine
4. aniline
An aromatic primary amine with cold nitrous acid leads to the formation of:
1. alcohol
2. nitrite
3. diazonium salt
4. benzene
X and Y in the given reaction are:

1.
2.
3.
4.
Which of the following statements about primary amines is false ?
[2010]
1. Alkyl amines are stronger bases than aryl amines
2. Alkyl amines react with nitrous acid to produce alcohols
3. Aryl amines react with nitrous acid to produce phenols
4. Alkyl amines are stronger bases than ammonia
Among the following, the strongest base is:
1. C6H5NH2
2. p-NO2-C6H4NH2
3. m-NO2-C2H4NH2
4. C2H5CH2NH2
Which of the following reactions does not yield an amine?
1. 
![]()
2. 

3. 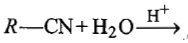
![]()
4. 

The end product in the following sequence of reactions is -
1. Ethyl cyanide
2. Ethylamine
3. Methylamine
4. Acetamide
Identfy X in the sequence,
:
1.
2.
3.
4. none of the above
CH3CH2NH2 contains a basic NH2 group, but CH3CONH2 does not, because;
1. acetamide is amphoteric in character
2. in CH3CH2NH2 the electron pair on N-atom is delocalized by resonance
3. in CH3CH2NH2 there is no resonance, while in acetamide the lone pair of electron on N-atom is delocalized and therefore less available for protonation
4. none of the above
Tertiary nitro compounds cannot show tautomerism because:
1. they are very stable
2. isomerises to give sec. nitro compounds
3. do not have labile H-atom
4. they are highly eactive






