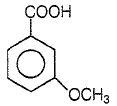
Which one of the following can be oxidised to the corresponding carbonyl compound?
1. 2-hydroxy propane
2. Ortho-nitro phenol
3. Phenol
4. 2-methyl-2-hydroxy propane
The -OH group of an alcohol or the -COOH group of a carboxylic acid can be replaced by -Cl using
1. phosphorus pentachloride
2. hypochlorous acid
3. chlorine
4. hydrochloric acid
Acetone reacts with iodine (I2) to form iodoform in the presence of
1. CaCO3
2. NaOH
3. KOH
4. MgCO3
A and B in the following reactions are


1. A=RR'CH2CN, B=NaOH
2. A=RR'C , B=CH3
3. A=RR'C , B=CH3
4. A=RR"C, B=LiAlH4
Acetaldehyde normally reacts with
1. only electrophiles
2. only nucleophiles
3. only free radicals
4. both electrophiles and nucleophiles
Nucleophilic addition reaction will be most favoured in [2006]
1.
2.
3.
4.
The enolic form of acetone contains:
1. 9 -bonds, 1-bond and 2 lone pairs
2. 8 -bonds, 2-bonds and 2 lone pairs
3. 10 -bonds, 1-bond and 1 lone pair
4. 9 -bonds, 2-bonds and 1 lone pair
In this reaction,
CH3CHO + HCN CH3CH(OH)CN CH3CH(OH)COOH
an asymmetric compound is generated. The acid obtained would be
1. 50% D + 50% L-isomer
2. 20% D + 80% L-isomer
3. D-isomer
4. L-isomer
Formaldehyde can be distinguished from acetaldehyde by:
1. Fehling's solution
2. Schiff's reagent
3. Ammonia
4. Ammoniacal


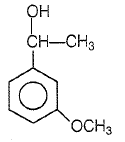
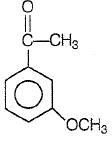
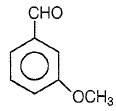
Product P in the above reaction is -
1. 

2. 

3. 

4.