The -OH group of an alcohol or the -COOH group of a carboxylic acid can be replaced by -Cl using
1. phosphorus pentachloride
2. hypochlorous acid
3. chlorine
4. hydrochloric acid
What ester produces a white crystalline acid when boiled with KOH, cooled, and acidified with conc. HCl?
1. Methyl acetate
2. Ethyl acetate
3. Ethyl formate
4. Ethyl benzoate
The compound formed when malonic acid heated with urea, is
1. cinnamic acid
2. butyric acid
3. barbituric acid
4. crotonic acid
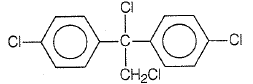
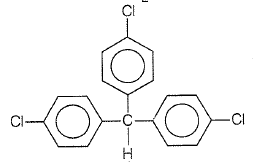
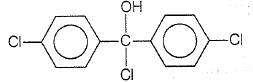
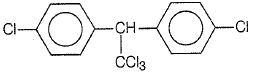
Trichloroacetaldehyde, CCl3CHO reacts with chlorobenzene in the presence of sulphuric acid and produces
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
1. 
2. 
3. 
4. 
What compound results from the catalytic dehydrogenation of a primary alcohol?
1. Aldehyde
2. Ketone
3. Alkene
4. Acid
The compound which on reduction with gives two diffrent alcohols:
1.
2.
3.
4.
HCHO and HCOOH are distinguished by treating with:
1. Tollens reagent
2. NaHCO3
3. Fehling's Solution
4. Benedict Solution
Lacrymator or tear gas is:
1. C6H5COCl
2. C6H5OC6H5
3. C6H5COCH2Cl
4. C6H5COCH3
Formaldehyde can be distinguished from acetaldehyde by:
1. Fehling's solution
2. Schiff's reagent
3. Ammonia
4. Ammoniacal
The correct acidity order of the following is:


1. (III)>(IV)>(II)>(I)
2. (IV)>(III)>(I)>(II)
3. (III)>(II)>(I)>(IV)
4. (II)>(III)>(IV)>(I)






