The hydrolysis of ester is alkaline medium is a
(1) 1st order reaction with molecularity 1
(2) 1 st order reaction with molecularity 2
(3) 2nd order reaction with molecularity 1
(4) 2nd order reaction with molecularity 2
The rate constant is given as \(K = 1.2 \times 10^3~mol^{-1} L^{-1} s^{-1}\), and the activation energy is \(E_a = 2.0 \times 10^2 ~kJ mol^{-1}\). As temperature T approaches infinity, what is the value of the pre-exponential factor (A)?
1. \(2.0 \times 10^2 \mathrm{~mol}^{-1} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
2. \(1.2 \times 10^3 \mathrm{~mol}^{-1} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
3. \(3.3 \times 10^3 \mathrm{~mol}^{-1} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
4. \(2.4 \times 10^3 \mathrm{~mol}^{-1} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
The reate constant for a reaction at 300K and at 340K. The
energy of activation of the reaction is
1. 14.695 KJ mol
2. 29.39 KJ mol
3. 44 KH mol
4. 22 KJ mol
For an endothermic reaction, where H represents the enthalpy of the reaction in KJ/mol, the minimum value for the energy of activation will be
1. Less the H
2. Zero
3. More than H
4. Equal to H
If the temperature coefficient of a reaction is 3, to what factor the rate of raction
increases increase when temperature is increased from 30 C to 60 C?
1. 3
2. 6
3. 9
4. 27
A first-order reaction is 15 % completed in 20 minutes. The amount of time required to complete 60 % of the reaction is:
| 1. | 112.8 min | 2. | 120.7 min |
| 3. | 100.4 min | 4. | 140.7 min |
Whcih of the following represents the expression for 3/4th life of a first order reaction?
1.
2.
3.
4.
In a catalytic reaction, involving the formation of ammonia by Haber's process the rate of appearance of NH3. was measured as . The rate of disappearance of H2 will be-
1. 2.5 10 mol L s
2. 1.25 10 mol L s
3. 3.75 10 molL s
4. 5.00 10 mol L s
For the zero-order reaction A B + C; initial concentration of A is 0.1 M. If A = 0.08 M after 10 minutes, then it's half-life and completion time are respectively:
1. 10 min; 20 min
2. 2x m; 4x min;
3. 25 min, 50 min
4. 250 min, 500 min
has a half life of 22 years. The decays follows two parallel paths
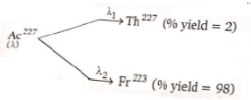
What are the decay constants () for Th and Fr respectively?
1. 0.03087, 0.00063
2. 0.00063, 0.03087
3. 0.02,0.98
4. None of these




