has a half life of 22 years. The decays follows two parallel paths
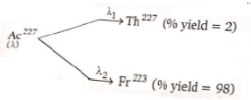
What are the decay constants () for Th and Fr respectively?
1. 0.03087, 0.00063
2. 0.00063, 0.03087
3. 0.02,0.98
4. None of these

A reaction takes place in various steps. The rate constant for first, second, third
and fifth steps are , , and respectively. The overall rate constant is
given by
If activation energy are 40, 60, 50 and 10 kJ/mol respectively, the overall
energy of activation (kJ/mol) is :
1. 10
2. 20
3. 25
4. None of these
The following reaction was carried out at 300 K.
2SO2(g) + O2(g) → 2SO3(g)
The rate of formation of is related to the rate of disappearance of by the following expression:
1.
2.
3.
4. None of the above.
Which of the following rate laws has an over all order of 0.5 for the reaction A + B + C product ?
1. R=k[A].[B].[C]
2. R=k[A]0.5[B]0.5[C]0.5
3. R=k[A]1.5[B]-1[C]0
4. R=k[A][B]0[C]0.5
For the system If the activation energy for the forward step is 100 kcal/mol.What is the ratio of temperature at which the forward and backward reaction shows the same % change of rate constant per degree rise of temperature ? (1 cal= 4.2 J)
(A)
(B)
(C)
(D)
For a reaction of the type 2A+B 2C, the rate of the reaction is given by . When the volume of the reaction vessel is reduced to 1/4 th of the original volume, the rate of reaction changes by a factor of
(A) 0.25
(B) 16
(C) 64
(D) 4
The rate constant of a first-order reaction is typically determined from a plot of:
1. Concentration of reactant vs time t
2. Log (concentration of reactant) vs time t
3. \(\frac{1}{\text { concentration of reactant }} \text { vs time } t\)
4. Concentration of reactant vs log time t
The plot of log k versus is linear with a slope of
(1)
(2)
(3)
(4)
The rate constant, the activation energy, and the Arrhenius parameter of a chemical reaction at 25°C are 3.0×10-4 s-1, 104.4 kJ mol-1 and 6.0×1014s-1 respectively.
The value of the rate constant as T → ∞
will be:
1. 2.0 × 1018 s-1
2. 6.0 × 1014 s-1
3.
4. 3.6 × 1030 s-1
87.5% of a radioactive substance disintegrates in 40 minutes. What is the half life of the substance ?
(1) 13.58 min
(2) 135.8 min
(3) 1358 min
(4) None of these




