In thermodynamics, a process is called reversible when:
1.
The system and surroundings change into each other.
2.
There is no boundary between the system and the surroundings.
3.
The surroundings are always in equilibrium with the system.
4.
The system does not change into the surroundings spontaneously.
;
which of the following is correct?
(1) ;
(2)
(3) Graphite is the stabler allotrope
(4) Diamond and graphite both are sp2 hybridised
Which of the following is not a correct statement? [ (Engg.) 2002]
1. When ΔG is negative, the process is spontaneous
2. When ΔG is zero, the process is in a state of equilibrium
3. When ΔG is positive, the process is non-spontaneous
4. None of these
The enthalpy of neutralization of HCN by NaOH is –12.13 kJ mol–1. The enthalpy of ionisation of HCN will be -
1. 45.07 kJ
2. 4.310 kJ
3. 451.9 kJ
4. 33.77 kJ
The heat liberated when 1.89 g of benzoic acid is burnt in a bomb calorimeter at 25°C increases the temperature of 18.94 kg of water by 0.632°C. If the specific heat of water at 25°C is 0.998 cal/g-deg, the value of the heat combustion of benzoic acid is
(1) 771.1 kcal
(2) 871.2 kcal
(3) 881.1 kcal
(4) 981.1 kcal
A Beckmann thermometer is used to measure -
1. High temperature
2. Low temperature
3. Normal temperature
4. All temperatures
Which of the following thermodynamic quantities is measured as heat in a bomb calorimeter during a reaction?
| 1. | ΔG | 2. | ΔH |
| 3. | ΔU | 4. | PΔV |
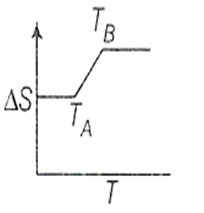
If for a given substance melting point is TB and freezing point is TA, then correct variation shown by graph between entropy change and temperature is [DCE 2001]
(1) 
(2)
(3)
(4)
In an isobaric process, the ratio of heat supplied to the system (dQ) and work done by the system (dW) for diatomic gas is:
1. 1 : 1
2. 7 : 2
3. 7 : 5
4. 5 : 7
When a gas undergoes adiabatic expansion, it gets cooled due to -
1. Loss of energy
2. Fall in pressure
3. Decrease in velocity
4. Increase in energy with work done









