A system is changed from state A to state B by one path and from B to A by another path. If E1 and E2 are the corresponding changes in internal energy, then [Pb. PMT 2001]
1.
2.
3.
4. None of the above
If (i) , (ii) , (iii) , the heats of reaction are Q, –12, –10 respectively. Then Q = [Orissa JEE 2004]
(1) – 2
(2) 2
(3) – 22
(4) – 16
Adsorption of gases on a solid surface is generally exothermic because [IIT JEE (Screening) 2004]
1. Enthalpy is positive
2. Entropy decreases
3. Entropy increases
4. Free energy increase
What is the enthalpy change (in kJ) for a process if two moles of an ideal gas are expanded isothermally and reversibly from 1 litre to 10 litre at 300 K?
1. 11.4 kJ
2. –11.4 kJ
3. 0 kJ
4. 4.8 kJ
When a gas undergoes adiabatic expansion, it gets cooled due to -
1. Loss of energy
2. Fall in pressure
3. Decrease in velocity
4. Increase in energy with work done
In an isobaric process, the ratio of heat supplied to the system (dQ) and work done by the system (dW) for diatomic gas is:
1. 1 : 1
2. 7 : 2
3. 7 : 5
4. 5 : 7
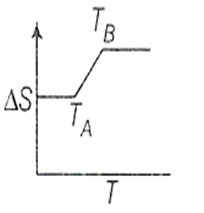
If for a given substance melting point is TB and freezing point is TA, then correct variation shown by graph between entropy change and temperature is [DCE 2001]
(1) 
(2)
(3)
(4)
Which of the following thermodynamic quantities is measured as heat in a bomb calorimeter during a reaction?
| 1. | ΔG | 2. | ΔH |
| 3. | ΔU | 4. | PΔV |
A Beckmann thermometer is used to measure -
1. High temperature
2. Low temperature
3. Normal temperature
4. All temperatures
The heat liberated when 1.89 g of benzoic acid is burnt in a bomb calorimeter at 25°C increases the temperature of 18.94 kg of water by 0.632°C. If the specific heat of water at 25°C is 0.998 cal/g-deg, the value of the heat combustion of benzoic acid is
(1) 771.1 kcal
(2) 871.2 kcal
(3) 881.1 kcal
(4) 981.1 kcal









