The appropriate reagent for the following transformation,
 is :
is :
1. Zn(Hg),HCl
2. NH2NH2, OH-
3. H2/Ni
4. NaBH4
 is :
is :Which compound would give 5-keto-2-methyl hexanal upon ozonolysis?
| 1. | 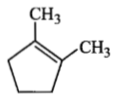 |
2. | 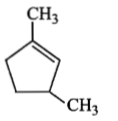 |
| 3. | 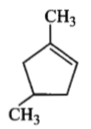 |
4. | 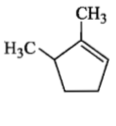 |
The reduction of an alkyne to alkene using Lindlar's catalyst results into:
1. Cis-addition of hydrogen atoms.
2. Trans-addition of hydrogen atoms.
3. A mixture obtained by cis- and trans-additions of hydrogen which are in equilibrium with each other.
4. A mixture obtained by cis- and trans-additions of hydrogen atoms which are not in equilibrium with each other.
Identify Z in the sequence:
\(\mathrm{CH}_ 3-\mathrm{CH}_2-\mathrm{CH}=\mathrm{CH}_2 \xrightarrow{\mathrm{HBr} / \mathrm{H}_2 \mathrm{O}_2} \mathrm{Y} \xrightarrow{\mathrm{C}_2 \mathrm{H}_5 \mathrm{O}^{-}\mathrm{Na}^{+}} \mathrm{Z} \)
| 1. |  |
| 2. |  |
| 3. | \( \mathrm{CH}_3-\left(\mathrm{CH}_2\right)_3-\mathrm{O}-\mathrm{CH}_2-\mathrm{CH}_3 \) |
| 4. | \( \mathrm{CH}_3-\left(\mathrm{CH}_2\right)_4-\mathrm{O}-\mathrm{CH}_3 \) |
Acetylene can be converted to higher alkyne using the following sequence of reactions:
(1) Na,RX
(2) RMg X,R X
(3) either of these two
(4) none of these
RCH2OH. This mode of reduction can be effected only by:
1. NaBH4
2. KMnO4
3. LiAIH4
4. All of the above
Propene, CH3-CH=CH2 can be converted into 1-propanol by oxidation. Which set of reagents among the following is ideal to effect the conversion?
1. Alkaline KMnO4
2. B2H6 and alk. H2O2
3. O3/zinc dust
4. OsO4/CHCl3
CH3CH=CHCHO is oxidized to CH3CH=CHCOOH using:
1. Alkaline permanganate
2. Ammoniacal silver nitrate
3. Selenium dioxide
4. Osmium tetroxide
An alkene, obtained by the dehydration of an alcohol(A), on ozonolysis gives two molecules of acetaldehyde for every molecule of alkene. the alcohol (A) is:
1.
2.
3.
4.
Propene on reaction with diazomethane in presence of UV radiations gives:
(1) cyclopropane
(2) methyl cyclopropane
(3) butane
(4) butene






