Which of the following compounds is most prone to oxidation?
1.
\(\mathrm{CH}_3-\mathrm{CHOH}-\mathrm{CH}_3\)2.

3.
\(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{O}-\mathrm{CH}_2-\mathrm{CH}_3\)4.
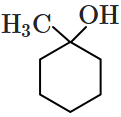
\(\mathrm{CH}_3-\mathrm{CHOH}-\mathrm{CH}_3\)

\(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{O}-\mathrm{CH}_2-\mathrm{CH}_3\)

Which of the following react with HBr at faster rate?
| 1. | \(\mathrm{NaBH}_4 \) | 2. | \(\mathrm{LiAlH}_4 \) |
| 3. | \(PCC \) | 4. | \(\mathrm{KMnO}_4\) |

| 1. |  |
2. |  |
| 3. |  |
4. |  |

(a)
(b)
(c)
(d)

(a) No reaction
(b)
(c)
(d)
Consider the following alcohols,
(I) 
(III)
The order of decreasing reactivities of these alcohols towards nucleophilic substitution with HBr is:-
(a) III>I>IV>II
(b) III>I>II>IV
(c) I>III>IV>II
(d) I>III>II>IV

Which is the best reagent to convert isopropyl alcohol to isopropyl bromide ?
(1) HBr
(2) SOBr2
(3) Br2
(4) CH3MgBr
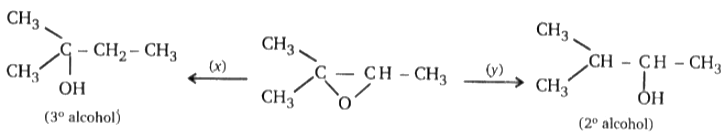
Find missing reagents.
1. x = LiAlH4, y = NaBH4
2. x = LiAIH4 / AlCl3, y = LiAlH4
3. x = LiAIH4, Y = LiAIH4 / AlCl3
4. x = H2 / Ni, y = H2 / Pt




















