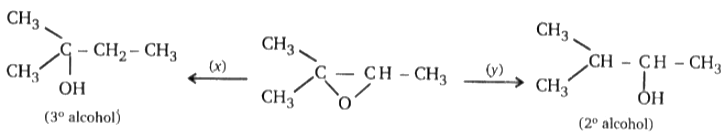

Find missing reagents.
1. x = LiAlH4, y = NaBH4
2. x = LiAIH4 / AlCl3, y = LiAlH4
3. x = LiAIH4, Y = LiAIH4 / AlCl3
4. x = H2 / Ni, y = H2 / Pt


1. x = LiAlH4, y = NaBH4
Which are not cleaved by HIO4?
I : glycerol
II : glycol
III: 1, 3-propanediol
IV: methoxy-2-propanol
1. I, II, III, IV
2. I, II
3. II, III
4. III, IV
Which of the following compounds are not oxidized by HIO4?
(1) 

(4) 


(7)
1. 5,6,7
2. 4,5,6,7
3. 6,7
4. 3,4,5,6,7
Succinic acid (A)(B)(C); Product (C) will be:
(1)
(2)
(3)
(4)

(C)(D) Product
(D) in above reaction Is:
(a)
(b)
(c)
(d)
(A) → B + C; (B) and (C) both gives +ve iodoform test.Compound (A) is:-
C5H10O H3O+
(1) CH3-CH=CH-O-CH2-CH3
H
l
(2)CH3-C-O-CH2-CH3
l
CH3
(3)CH3-C-O-CH2-CH3
ll
CH2
(4)Both (b) and (c)
A solution of Ph3CCO2 in conc. H2SO4 gives (X) when poured into methanol X is :-
O
ll
(1) Ph3C-C-O-CH3
O
ll
(2) Ph2CH-C-O-CH3
(3) Ph3C-OCH3
(4) Ph3C-CH3
The reducing agent among the following is -
1. CrO3/H+
2. KMnO4
3. LiAlH4
4. O3
Products obtained in the above reaction are :
(1) HCHO, HCO2H
(2) HCHO, 2HCO2H
(3) CO2, 2HCO2H
(4) CO2, HCHO, HCO2H
0.092 g of a compound with the molecular formula C3H8O3 reaction with an excess of CH3MgI gives 67.00 mL of methane at STP. The number of active hydrogen atoms present in a molecule of the compound is:
(1) one
(2) two
(3) three
(4) four












