Upon monochlorination of the hydrocarbon (as given in the reaction below), the number of chiral centres generated is/are:

1. 3
2. 4
3. 1
4. 2
1. 3
2. 4
3. 1
4. 2
\(\mathrm{CH}_3 \mathrm{Cl} \longrightarrow \mathrm{CH}_4\)
1. \(\mathrm{Zn} / \mathrm{H}^{+}\)
2. \(\mathrm{LiAlH}_4\)
3. \(\mathrm{Mg}\text{/(ether) then } \mathrm{H}_2 \mathrm{O}\)
4. All of the above

| 1. |  |
2. |  |
| 3. |  |
4. | Both (2) & (3) |
Ethane is subjected to combustion process. During the combustion the hybrid state of carbon changes from:-
(1) sp2 to sp3
(2) sp3 to sp
(3) sp to sp3
(4) sp2 to sp2
The above reaction is an example of:-
1. isomerization
2. polymerization
3. cracking
4. de-hydrogenation
Consider the following compounds:
(I) 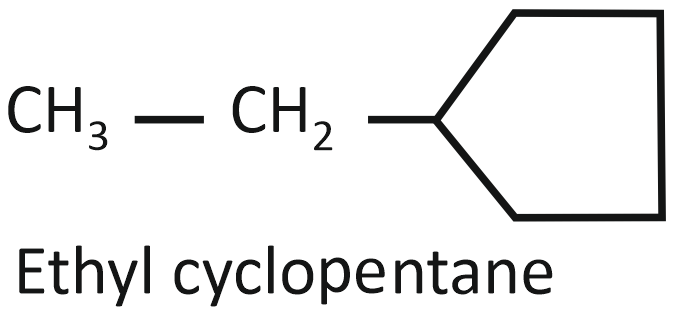
(II)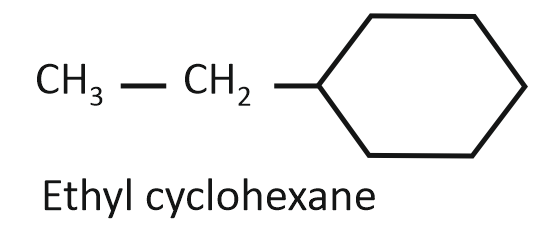
(III) 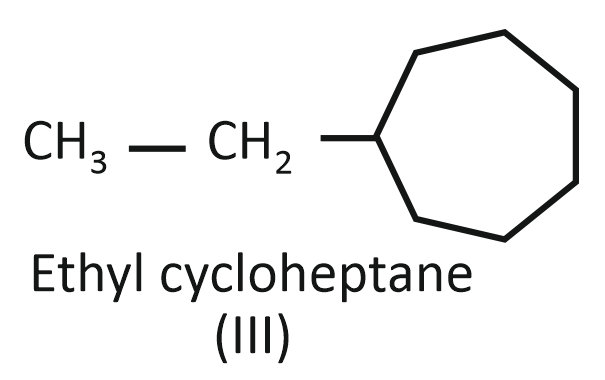
Arrange the compounds I, II, and III in decreasing order of their heats of combustion:
1. II>I>III
2. I>II>III
3. III>II>I
4. III>I>II
Which substrate is appropriate for the preparation of methane and ethane in a single-step process?
1. H2C = CH2
2. CH3OH
3. CH3-Br
4. CH3-CH2-OH

The number of possible monobromo products is (excluding stereoisomers):
1. 4
2. 5
3. 8
4. 10

1. \(\mathrm{CH}_3-\mathrm{CH}_3\)
2. \(\mathrm{CH}_2=\mathrm{CH}_2\)
3. \(\mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}_2\)
4. None of the above.












