
The above reaction is an example of:-
1. isomerization
2. polymerization
3. cracking
4. de-hydrogenation

The above reaction is an example of:-
1. isomerization
Consider the following compounds:
(I) 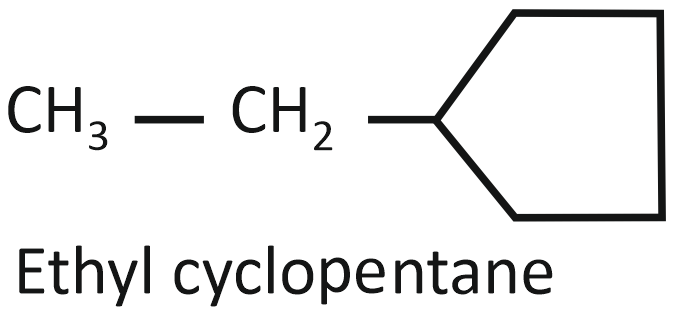
(II)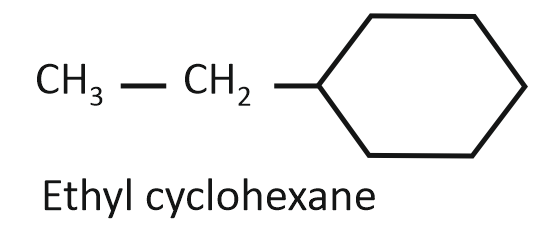
(III) 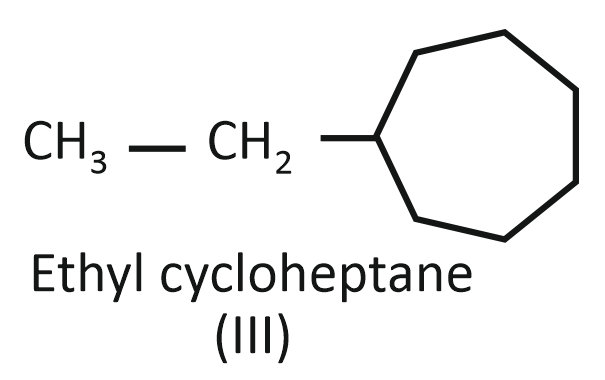
Arrange the compounds I, II, and III in decreasing order of their heats of combustion:
1. II>I>III
2. I>II>III
3. III>II>I
4. III>I>II
Which substrate is appropriate for the preparation of methane and ethane in a single-step process?
1. H2C = CH2
2. CH3OH
3. CH3-Br
4. CH3-CH2-OH

The number of possible monobromo products is (excluding stereoisomers):
1. 4
2. 5
3. 8
4. 10

1. \(\mathrm{CH}_3-\mathrm{CH}_3\)
2. \(\mathrm{CH}_2=\mathrm{CH}_2\)
3. \(\mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}_2\)
4. None of the above.
Which of the following bromides is the major product of the reaction shown below, assuming that there are no carbocation rearrangement?
1.
2.
3.
4.
What is a likely product of the reaction shown?
1.
2.
3.
4.
Which of the following, when undergoing addition of HBr, will form ONLY a pair of diastereomers?
Which of the following is the best stereochemical representation when reaction between 1 -methylcyclohexene and NBS react in aqueous dimethyl sulfoxide?
| 1. |  |
2. |  |
| 3. |  |
4. | None of these |






















