Select Question Set:
In the presence of sunlight, benzene reacts with \(Cl_2\) to give product 'X'. The number of hydrogens in 'X' is:
1. Five (5)
2. Three (3)
3. Six (6)
4. Twelve (12)
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
68%
Level 2: 60%+
Please attempt this question first.
Hints
Please attempt this question first.
Given below are two statements:
| Statement I: | The rate of reaction of alkanes with halogens in the presence of light is I2 > Br2 > Cl2 > F2 |
| Statement II: | Iodination of an alkane is carried out in the presence of oxidizing agents like HlO3 or HNO3 |
| 1. | Both Statement I and Statement II are correct. |
| 2. | Both Statement I and Statement II are incorrect. |
| 3. | Statement I is correct but Statement II is incorrect. |
| 4. | Statement I is incorrect but Statement II is correct. |
Subtopic: Alkanes, Alkenes and Alkynes - Chemical Properties |
60%
Level 2: 60%+
Please attempt this question first.
Hints
Please attempt this question first.
Which of the following is correct statement?
| 1. | Cis-but-2-ene has a higher boiling point than Trans-but-2-ene. |
| 2. | Cis-but-2-ene has a lower boiling point than Trans-but-2-ene. |
| 3. | Cis-but-2-ene has a lower dipole moment than Trans-but-2-ene. |
| 4. | Cis-but-2-ene is non-polar. |
Subtopic: Alkanes, Alkenes and Alkynes - Chemical Properties |
67%
Level 2: 60%+
Please attempt this question first.
Hints
Please attempt this question first.
Consider the following sequence of reactions and select the correct option for the product:
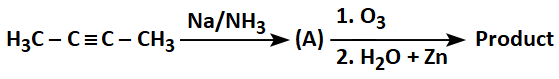
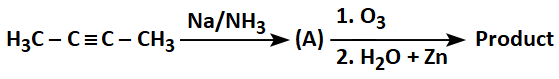
| 1. | 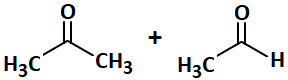 |
| 2. | 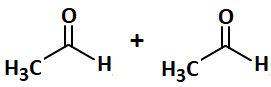 |
| 3. | 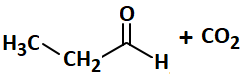 |
| 4. | 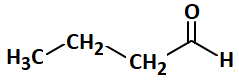 |
Subtopic: Alkanes, Alkenes and Alkynes - Chemical Properties |
87%
Level 1: 80%+
Please attempt this question first.
Hints
Please attempt this question first.
Which of the following molecules contains 1°, 2°, and 3° carbon atoms?
1. 2,3,4-Trimethylpentane
2. Chlorocyclohexane
3. 1,1-Dimethylcyclohexane
4. Methylcyclohexane
1. 2,3,4-Trimethylpentane
2. Chlorocyclohexane
3. 1,1-Dimethylcyclohexane
4. Methylcyclohexane
Subtopic: Aliphatic Hydrocarbon -Nomenclature, Isomerism & Mechanism |
60%
Level 2: 60%+
Please attempt this question first.
Hints
Given below are two statements:
| Assertion (A): | Alkenes and series of cycloalkanes of hydrocarbons have the same general formula. |
| Reason (R): | Either insertion of a double bond or formation of a ring reduces the number of hydrogen atoms of the corresponding alkane by 2. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
Subtopic: Aliphatic Hydrocarbon -Nomenclature, Isomerism & Mechanism |
79%
Level 2: 60%+
Hints
Identify the staggered conformation with maximum dihedral angle:
| 1. | 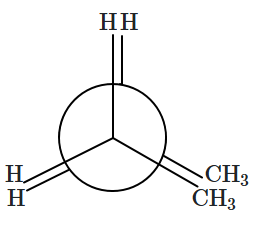 |
2. |  |
| 3. |  |
4. |  |
Subtopic: Alkanes, Alkenes and Alkynes - Chemical Properties |
84%
Level 1: 80%+
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Match the bond line structures of hydrocarbons given in List-I with the corresponding boiling points given in List-II.
| List-I (Compound) | List-II (Boiling point in K) | ||
| (a) |  |
(i) | 300.9 |
| (b) |  |
(ii) | 282.5 |
| (c) |  |
(iii) | 309.1 |
| (d) |  |
(iv) | 341.9 |
Choose the correct answer from the options given below:
| (a) | (b) | (c) | (d) | |
| 1. | (i) | (iv) | (iii) | (ii) |
| 2. | (iii) | (i) | (iv) | (ii) |
| 3. | (iii) | (iv) | (i) | (ii) |
| 4. | (iv) | (i) | (ii) | (iii) |
Subtopic: Aliphatic Hydrocarbon- Physical Properties |
83%
Level 1: 80%+
NEET - 2022
Hints
Compound X on reaction with \(O_3\) followed by \(\mathrm{Zn/ H_2O}\) gives formaldehyde and 2-methyl propanal as products. The compound X is:
| 1. | Pent-2-ene | 2. | 3-Methylbut-1-ene |
| 3. | 2-Methylbut-1-ene | 4. | 2-Methylbut-2-ene |
Subtopic: Alkanes, Alkenes and Alkynes - Chemical Properties |
73%
Level 2: 60%+
NEET - 2022
Hints
Which of the following compounds does not exhibit aromaticity?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
75%
Level 2: 60%+
NEET - 2022
Hints
Select Question Set:






