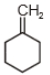
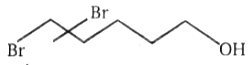
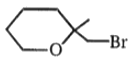
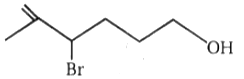
The major product in the acid catalysed dehydration of 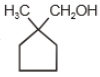 would be
would be
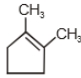
1. 
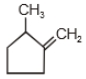
2. 
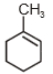
3. 
4. 
 would be
would be



Which of the following reactions will give 2° chiral alcohol as one or more of major organic products?
| (1) |  |
| (2) |  |
| (3) |  |
| (4) | None |
The major product of the following reaction sequence will be
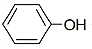
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
In the following reactions X, Y and Z are respectively :
| X | Y | Z | |
| (1) |  |
 |
 |
| (2) |  |
 |
 |
| (3) |  |
 |
 |
| (4) |  |
 |
 |
An aromatic compound A (C8H10O) gives the following tests with the given reagents.
The structure of 'A' is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Compound Y, C7H8O is insoluble in water, dil HCI and aqueous NaHCO3. It dissolves in dilute NaOH. When Y is treated with bromine water it is converted rapidly into a compound of formula C7H5OBr3. The structure of Y is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Compounds I and II can be distinguished by using reagent:
(I) 4–Amino–2–methylbut–3–en–2–ol
(II) 4–Amino–2,2–dimethylbut–3–yn–1–ol
1. NaNO2/HCl
2. Br2/H2O
3. HCl/ZnCl2 (anhydrous)
4. Cu2Cl2 + NH4OH
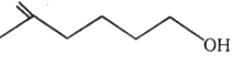

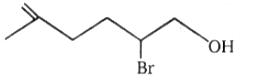
1. 
2. 
3. 
4. 
What is the correct order of dehydration rate for the compounds (i), (ii), and (iii) when treated with concentrated \(\text{H}_2\text{SO}_4\)?
| (i) |  |
| (ii) |  |
| (iii) |  |
1. (i) > (iii) > (ii)
2. (i) > (ii) > (iii)
3. (ii) > (i) > (iii)
4. (ii) > (iii) > (i)
Product (x) will be:
(a)
(b)
(c)
(d)

















