Which reagent distinguish propyne and propene?
1. Alkaline
2. +
3. water
4. +
XY
Y is:
1. 
3. 4.
The alkene among the following that has the smallest heat of hydrogenation is-
1.
2.
3.
4.
Which of the following alkanes will give the maximum number of isomers when a monochloro-substituted product is obtained?
| 1. |  |
2. |  |
| 3. | \(\mathrm{CH_4}\) |
4. |  |
The correct order of relative rates of hydrogenation of alkenes is:
1. Ethylene > propene > 2-butene > 2-methyl-2-butene
2. 2-methyl-2-butene > 2-butene > Propene > Ethylene
3. 2-butene > propene > ethylene > 2-methyl-2-butene
4. Propene > 2-butene > ethylene > 2-methyl-2-butene
Consider the given reaction:
 Products formed
Products formed
| (A) |  |
(B) |  |
| (C) |  |
(D) |  |
1. (A) and (B) only 2. (A) and (C) only
3. (B) and (C) only 4. (A), (B), (C) and (D)
An unsaturated hydrocarbon on complete hydrogenation gives 1-isopropyl-3 methylcyclohexane, after ozonolysis it gives one mole of formaldehyde, one mole of acetone and one mole of 2,4-Dioxohexanedial. The possible structure\s of the hydrocarbon may be
1. 
2. 
3. 
4.
Which one of the following reactions is not possible?
1. CH3COONa + HCI → CH3COOH + NaCI
2. CH3–SO3H + → CH3SONa +
3. + PhONa → PhOH +
4. + NaNH2→
+ NH3
Observe the following sequence of reactions.

The product R is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
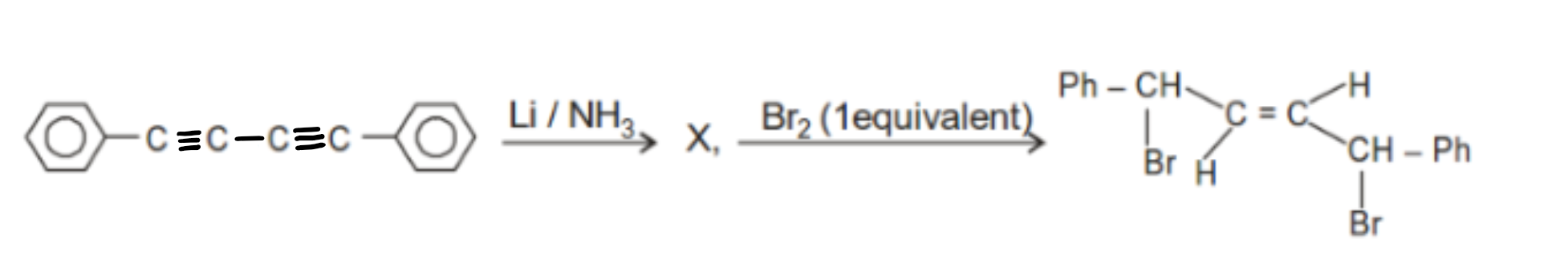
'X' is :
| (1) |  |
| (2) |  |
| (3) | Both |
| (4) | None of these |







