The ligand(s)that show a chelating effect is/are:
1.
Diethylenetriamine
2.
Oxalato
3.
Both 1 and 2
4.
None of these
Which of the following statements is incorrect?
| 1. | Chlorophyll is a coordination compound of magnesium. |
| 2. | Cis- Platin is a coordination compound of Platinum. |
| 3. | Gold is extracted by the addition of zinc metal. |
| 4. | None of the above. |
Optical isomerism is exhibited by among the following is/are:
| 1. | [Cr(Ox)3]3- | 2. | [PtCl2(en)2]2+ |
| 3. | [Cr(NH3)2Cl2en]+ | 4. | All of the above |
The incorrect statement among the following is:
| 1. | In a coordination entity, the central atom or ion is surrounded by a suitable number of neutral molecules or negative ions called ligands. |
| 2. | The coordination number is also termed ligancy. |
| 3. | A coordination polyhedron is the spatial arrangement of the ligands that are directly attached to the central metal ion in the coordination sphere. |
| 4. | None of the above. |
The correct statement(s) among the following options is/are :
1. Complexes in which the metal ion is bound to only one kind of a donor group are homoleptic complexes.
2. Heteroleptic complexes are those complexes where the central metal ion is bound to only one type of donor group.
3. is an example of a homoleptic complex.
4. Both (1) and (3)
Ethylene diaminetetraacetate (EDTA) ion is:
| 1. | Bidentate ligand with two "N" donor atoms |
| 2. | Tridentate ligand with three "N" donor atoms |
| 3. | Hexadentate ligand with four "O" and two "N'' donor atoms |
| 4. | Unidentate ligand |
Match Column-I with Column-II, Columns I and II represent complexes and magnetic moment (BM) respectively.
| Column-I | Column-II | ||
| (a) | [FeCN6]3- | (i) | 5.92 BM |
| (b) | [Fe(H2O)6]3+ | (ii) | 0 BM |
| (c) | [Fe(CN)6]4- | (iii) | 4.90 BM |
| (d) | [Fe(H2O)6]2+ | (iv) | 1.73 BM |
Choose the correct answer from the options given below:
| (a) | (b) | (c) | (d) | |
| 1. | (i) | (iii) | (iv) | (ii) |
| 2. | (iv) | (i) | (ii) | (iii) |
| 3. | (iv) | (ii) | (i) | (iii) |
| 4. | (ii) | (iv) | (iii) | (i) |
The correct splitting diagram of d orbitals in an octahedral crystal field is:
| 1. |  |
2. | 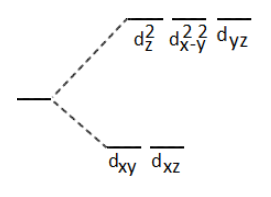 |
| 3. |  |
4. | None of the above |
The correct representation of potassium tetracyanidonickelate(II) is :
The correct representation of pentaamminenitrito-O-cobalt(III) is :






