For a reaction, 2A + B → C + D, the following observations were recorded:
Experiment
[A]/mol L–1
[B]/mol L–1
Initial rate of formation
of D/mol L–1 min–1
I
0.1
0.1
6.0 × 10–3
II
0.3
0.2
7.2 × 10–2
III
0.3
0.4
2.88 × 10–1
IV
0.4
0.1
2.40 × 10–2
The rate law applicable to the above mentioned reaction would be:
1. Rate = k[A]2[B]3
2. Rate = k[A][B]2
3. Rate = k[A]2[B]
4. Rate = k[A][B]
of D/mol L–1 min–1
Given the following observations:
| Experiment | [A] / mol L–1 | [B] / mol L–1 | Initial rate / mol L–1 min–1 |
| I | 0.1 | 0.1 | 2.0 × 10–2 |
| II | X | 0.2 | 4.0 × 10–2 |
| III | 0.4 | 0.4 | Y |
The reaction between A and B is first-order with respect to A and zero-order with respect to B. The values of X and Y are, respectively:
1. X = 0.2 \(mol\) \(L^{- 1}\); Y = \(\) \(0 . 08\) \(mol\) \(L^{- 1} \left(min\right)^{- 1}\)
2. X = 0.02 \(mol\) \(L^{- 1}\); Y = \(\) \(0 . 08\) \(mol\) \(L^{- 1} \left(min\right)^{- 1}\)
3. X = 0.01 \(mol\) \(L^{- 1}\); Y = \(\) \(0 . 8\) \(mol\) \(L^{- 1} \left(min\right)^{- 1}\)
4. X = 0.2 \(mol\) \(L^{- 1}\); Y = \(\) \(0 . 8\) \(mol\) \(L^{- 1} \left(min\right)^{- 1}\)
A radioactive substance has a rate constant of \(4 \, \text{years}^{-1}\). What is its half-life?
1. 0.05 years
2. 0.17 years
3. 0.26
4. 1.6 years
During a nuclear explosion, one of the products is 90Sr with a half-life of 28.1 years. If 1µg of 90Sr was absorbed in the bones of a newly born baby instead of calcium, the amount of 90Sr that will remain after 10 years in the now grown up child would be -
(Given ,antilog(0.108)=1.28)
1. 0.227 µg
2. 0.781 µg
3. 7.81 µg
4. 2.27 µg
For the decomposition of azoisopropane to hexane and nitrogen at 543 K, the following data was obtained:
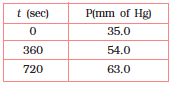
The rate constant of the above reaction would be -
| 1. | 1.21 × 10–3 s–1 | 2. | 2.21 × 10–3 s–1 |
| 3. | 3.21 × 10–3 s–1 | 4. | 4.21 × 10–3 s–1 |
For the reaction , rate = with k = and . The initial rate of the reaction will be :
1. 0.04 mol
2. 8
3. 8
4. 8 mol
For a first-order reaction, the relationship between time required for 99% completion to the time required for the completion of 90% of the reaction would be :
| 1. | 2. | ||
| 3. | 4. |
What are the dimensions of the rate constant K in the rate law \(\text { Rate }=k\left[H_2 O_2\right]\left[I^{-}\right]\)?
| 1. | 2. | ||
| 3. | 4. |
Consider the following rate expression.
The order of reaction and dimension of the rate constant are, respectively-
1. ; k =
2. 3; k =
3. ; k =
4. ; k =
If the concentration of the reactant is made twice, the new rate of reaction for the second order reaction would be-
1. 2 times
2. 4 times
3. 3 times
4. No change in the rate of the reaction






