The correct IUPAC name of the following structure is

1.
2,4,7-Trimethyloctane
2.
2,5,7-Trimethyloctane
3.
2,3,7-Trimethyloctane
4.
All of the above
The IUPAC name of the following molecule is -

| 1. | 2-Chloro-4-methylpentane | 2. | 4-Chloro-2-methylpentane |
| 3. | 4-Chloro-4-methylpentane | 4. | 2-Chloro-2-methylpentane |
The species present below is
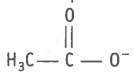
1. carbocation
2. electrophile
3. nucleophile
4. carboanion
ion is a-
1. carbocation
2. nucleophile
3. electrophile
4. carboanion
ion is a -
1. carbocation
2. carboanion
3. electrophile
4. nucleophile
molecule is -
1. carbocation
2. nucleophile
3. electrophile
4. carboanion
The species present below is
1. nucleophile
2. carbocation
3. electrophile
4. carboanion
Chlorine atom can be classified as:
| 1. | Carbocation | 2. | Nucleophile |
| 3. | Electrophile | 4. | Carbanion |
The correct hybridization states of carbon atoms in the above compound are -
| 1. | C1= sp , C2= sp3 | 2. | C1= sp2 , C2= sp |
| 3. | C1= sp3 , C2= sp | 4. | C1= sp , C2= sp2 |
The correct hybridization states of carbon atoms marked as 1,2,3 in the following compound are:
\({ }^1 \mathrm{CH}_3-\mathrm{ }^2{C} \mathrm{H}={}^3{\mathrm{C}} \mathrm{H}_2 \)
| 1. | C1= sp , C2= sp3 , C3= sp2 | 2. | C1= sp2 , C2= sp3 , C3= sp3 |
| 3. | C1= sp3 , C2= sp2 , C3= sp2 | 4. | C1= sp3 , C2= sp3 , C3= sp3 |






