Toluene on reaction with N-Bromosuccinimide gives
1. Phenyl bromomethane
2. o-Bromomethyl benzene
3. p-Bromomethyl benzene
4. m-Bromomethyl benzene
On sulphonation of C6H5Cl, the product obtained is-
1. m-Chlorobenzene sulphonic acid is formed
2. Benzene sulphonic acid is formed
3. o-Chlorobenzene sulphonic acid is formed
4. o-and p-Chlorobenzene sulphonic acid is formed
Propene upon heating with \(\mathrm{Cl}_2 \text { at } 500{ }^{\circ} \mathrm{C}\) forms:
| 1. | \(\mathrm{CH}_3-\mathrm{CHCl}-\mathrm{CH}_2 \mathrm{Cl} \) | 2. | \(\mathrm{CH}_2 \mathrm{Cl}-\mathrm{CH}=\mathrm{CH}_2 \) |
| 3. | \(\mathrm{CH}_2 \mathrm{Cl}-\mathrm{CHCl}-\mathrm{CH}_2 \mathrm{Cl} \) | 4. | All of the above |
Addition of KI accelerates the hydrolysis of primary alkyl halides because:
1. KI is soluble in organic solvents
2. The iodide ion is a weak base and a poor leaving group
3. The iodide ion is a strong base
4. The iodide ion is a powerful nucleophile as well as a good leaving group

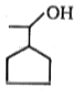
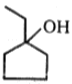
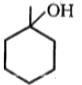
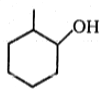
Major product (A) is:
1. 
2. 
3. 
4. 
What would be the major product of the given reaction?
1.
2.
3.
4.
In which reaction product formation does not take place by Hofmann's Rule?
1.
2.
3.
4.
Which of the following compound would not give an E2 product when treated with sodium ethoxide?
1.
2.
3.
4.
Which of the following is not a product of the above reaction?
1.
2.
3.
4.

























