Propene upon heating with \(\mathrm{Cl}_2 \text { at } 500{ }^{\circ} \mathrm{C}\) forms:
1.
\(\mathrm{CH}_3-\mathrm{CHCl}-\mathrm{CH}_2 \mathrm{Cl} \)
2.
\(\mathrm{CH}_2 \mathrm{Cl}-\mathrm{CH}=\mathrm{CH}_2 \)
3.
\(\mathrm{CH}_2 \mathrm{Cl}-\mathrm{CHCl}-\mathrm{CH}_2 \mathrm{Cl} \)
4.
All of the above
On sulphonation of C6H5Cl, the product obtained is-
1. m-Chlorobenzene sulphonic acid is formed
2. Benzene sulphonic acid is formed
3. o-Chlorobenzene sulphonic acid is formed
4. o-and p-Chlorobenzene sulphonic acid is formed
Toluene on reaction with N-Bromosuccinimide gives
1. Phenyl bromomethane
2. o-Bromomethyl benzene
3. p-Bromomethyl benzene
4. m-Bromomethyl benzene
Addition of KI accelerates the hydrolysis of primary alkyl halides because:
1. KI is soluble in organic solvents
2. The iodide ion is a weak base and a poor leaving group
3. The iodide ion is a strong base
4. The iodide ion is a powerful nucleophile as well as a good leaving group

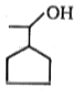
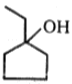
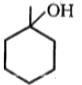
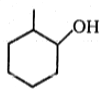
Major product (A) is:
1. 
2. 
3. 
4. 
What would be the major product of the given reaction?
1.
2.
3.
4.
In which reaction product formation does not take place by Hoffmann's Rule?
| 1. | 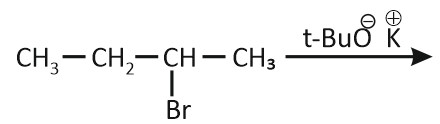 |
| 2. | 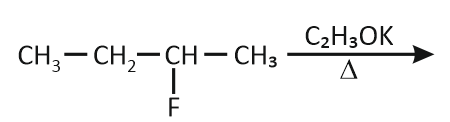 |
| 3. | 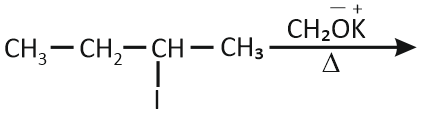 |
| 4. | 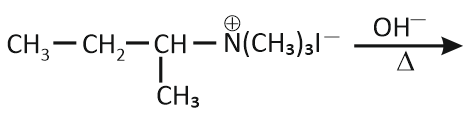 |
Which of the following compound would not give an E2 product when treated with sodium ethoxide?
1.
2.
3.
4.
Which of the following is not a product of the above reaction?
1.
2.
3.
4.

























