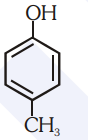
Which of the following substituted phenols is the strongest acid?
1.
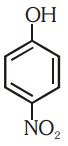

2.
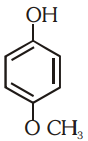
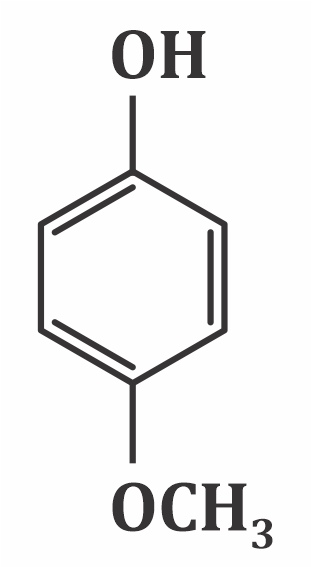
3.
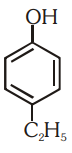

4.









When benzenediazonium chloride is treated with water, the compound formed is :
(1)
(2)
(3)
(4)
Cyclohexanol gives best yield of cyclohexene with -
1.
2. conc.HCl
3. conc.HBr
4. All of the above
| 1. | sec-Butyl alcohol | 2. | tert-Butyl alcohol |
| 3. | iso-Butyl alcohol | 4. | iso-Propyl alcohol |
Which of the following is produced when anisole reacts with HI?
| 1. | 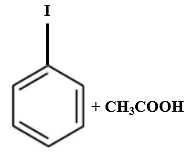 |
2. | 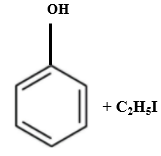 |
| 3. | 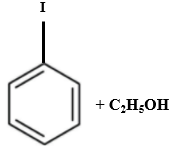 |
4. | 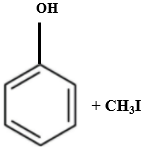 |
Preparation of phenol from cumene is -
1. Reduction Reaction
2. Oxidation reaction
3. Disproportionation Reaction
4. Ozonolysis
The reagent used for the bromination of phenol to 2,4,6-tribromophenol is/are:
1. Bromine water
2. Br2, CS2
3. Br2, CCl4
4. AgBr
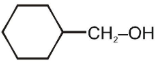
Amongst the following, the alcohol that reacts with conc. HCl /anhydrous ZnCl2 to form alkyl halide at room temperature, will be
1.
2.
3.
4. 
Match the structures of the compounds given in Column I with the name of the compounds given in Column II.
| Column I(Structure) | Column II(Name) | ||
| A. |  |
(i). | Phenetole |
| B. |  |
(ii). | o-Cresol |
| C. |  |
(iii). | Catechol |
| D. |  |
(iv). | Resorcinol |
Codes:
| A | B | C | D | |
| 1. | (ii) | (iv) | (i) | (iii) |
| 2. | (iii) | (i) | (iv) | (ii) |
| 3. | (i) | (iv) | (iii) | (ii) |
| 4. | (iv) | (iii) | (ii) | (i) |
Match the starting material in Column I with the products formed (Column II) in the reaction with HI.
| Column I |
Column II |
||
| A. |  |
1. |  |
| B. |  |
2. |  |
| C. |  |
3. |  |
| D. |  |
4. |  |
| 5. |  |
| A | B | C | D | |
| 1. | 2 | 3 | 4 | 1 |
| 2. | 3 | 1 | 5 | 2 |
| 3. | 5 | 4 | 3 | 2 |
| 4. | 4 | 5 | 2 | 1 |






