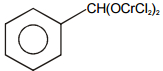
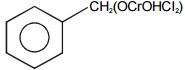
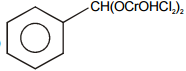
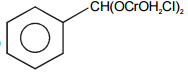
Select the structure of chromium complex formed. When the toluene reacts with chromyl chloride to give benzaldehyde on hydrolysis :
1. 
2. 
3. 
4. 




The product 'B' in the below mentioned reaction is:
\(CH_3 C \equiv N + H_2O \xrightarrow[\text{Excess}]{H^+}\ A\ \xrightarrow[\Delta]{NaOH\ +\ CaO}\ B\)
1.
2.
3.
4.
The reagent A in the below mentioned reaction is:
1. (i) LiAlH4; (ii) H2O
2. H2/Ni
3. (i) NaBH4; (ii) H2O
4. Zn-Hg/HCl
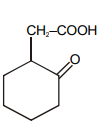
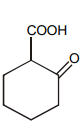
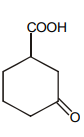
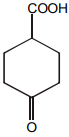
Which of the following is maximum reactive for decarboxylation :-
1. 
2. 
3. 
4. 
The reaction between benzaldehyde and acetophenone in the presence of dilute NaOH is known as:
1. Cannizzaro's reaction
2. Cross Cannizzaro's reaction
3. Cross aldol condensation
4. Aldol condensation
The product 'X' in the below mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Select the correct option based on statements below:
| Assertion (A): | Esterification of carboxylic acids with alcohol is a nucleophilic acyl substitution. |
| Reason (R): | Both the C-O bond lengths in HCOO- are equal. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
Consider the given two statements:
| Assertion (A): | Aldehydes are more reactive than ketones in nucleophilic addition reactions. |
| Reason (R): | The magnitude of the positive charge present on the carbonyl carbon of aldehydes is greater than that of ketones. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
Select the correct option based on statements below:
| Assertion (A): | The addition of ammonia derivatives to carbonyl compounds is carried out in a weakly acidic medium. |
| Reason (R): | In a weakly acidic medium, the attacking nucleophile is also protonated. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
Select the correct option based on statements below:
| Assertion (A): | Benzaldehyde undergoes a condensation reaction when heated in the presence of KCN. |
| Reason (R): | Benzaldehyde has no α- H atom. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |








