The reaction between benzaldehyde and acetophenone in the presence of dilute NaOH is known as:
1. Cannizzaro's reaction
2. Cross Cannizzaro's reaction
3. Cross aldol condensation
4. Aldol condensation
The product 'X' in the below mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Which substance when boiled with NaOH will evolve ?
(1) Ethylamine
(2) Aniline
(3) Acetamide
(4) Acetoxime
Which of the following enzymes can hydrolyse urea into CO2 and NH3:
1. Amylase
2. Urease
3. Lipase
4. Zymase
Identify product 'B' in the given reaction:

| 1. |  |
2. |  |
| 3. |  |
4. |  |
HCN not produce a chiral compound with:
| 1. | 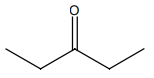 |
2. | 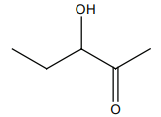 |
| 3. | 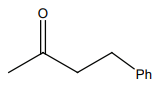 |
4. | 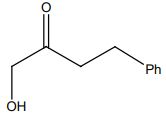 |
An alkene “A” on reaction with O3 and Zn - H2O gives propanone and ethanal in an equimolar ratio. The addition of HCl to alkene “A” gives “B” as the major product. The structure of product “B” is:
| 1. | 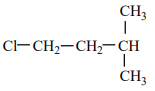 |
2. | 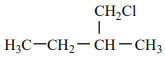 |
| 3. | 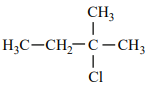 |
4. | 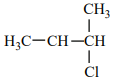 |
Carbonyl compounds undergo nucleophilic addition because of
1. The electrophilic carbonyl carbon forms a sigma bond with the nucleophile.
2. Electromeric effect.
3. Electronegativity difference of carbon and oxygen atoms.
4. None of the above
Carboxylic acids are more acidic than phenol and alcohol because of
1. formations of dimers
2. highly acidic hydrogen
3. resonance stabilization of their conjugate base
4. intermolecular hydrogen bonding
Acetaldehyde cannot exhibit
1. Iodoform test
2. Benedict's test
3. Lucas test
4. Tollen's test







