

can be repared from williamson's synthesis using
| 1. |  |
| 2. |  |
| 3. |  |
| 4. | All of these |
1. 
2. C6H5CH2Cl and
3. 
4. All of these
Methanol and ethanol can be distinguished by the following:
1. By reaction with metallic sodium
2. By reaction with caustic soda
3. By heating with iodine and washing soda
4. By heating with zinc and inorganic mineral acid
The product 'D' in the below mentioned reaction is:
\(CH_3MgBr + \text{cyclopentanone}\ \)
\(\xrightarrow{H_2O}\ A\ \xrightarrow{HBr}\ B\)
\( B\ \xrightarrow{Mg/Ether}\ C \xrightarrow[H_3O^+]{HCHO}\ D\)
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Consider the following alcohols,
| I. |  |
| II. |  |
| III. |  |
| IV. |  |
Ease of dehydration of the above alcohols will be in the order of:
| 1. | I < II < III < IV | 2. | I > II > III > IV |
| 3. | III < II < I < IV | 4. | II < III < IV < I |
The number of moles of HI
consumed by the given compound is:
| 1. | 1 | 2. | 2 |
| 3. | 3 | 4. | 4 |
R-CH2-CH2OH can be converted into RCH2CH2COOH. The correct sequence of reagents is:
1. PBr3, KCN, H+
2. PBr3, KCN, H2
3. KCN, H+
4. HCN, PBr3, H+
The strongest acid among the following aromatic compounds is -
| 1. | p-Chlorophenol | 2. | p-Nitrophenol |
| 3. | m-Nitrophenol | 4. | o- Nitrophenol |

The product (x) of this reaction is
1. 
2.
3.
4.
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
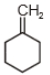
The major product in the acid catalysed dehydration of 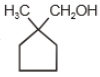 would be
would be
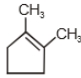
1. 
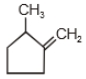
2. 
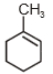
3. 
4. 
Chlorobenzene reacts with Mg in dry ether to give a compound (A) which further reacts
with ethanol to yield
1. phenol
2. benzene
3. ethyl benzene
4. phenyl ether












