The bond order for a species with the configuration will be:
1. 1
2.
3. Zero
4. \(\frac{3}{2}\)
Subtopic: M.O.T |
86%
Level 1: 80%+
Hints
Which of the following compounds has the least tendency to form hydrogen bonds between molecules?
1.
2.
3. HF
4.
Subtopic: van der Waal Force & Hydrogen Bonding |
Level 3: 35%-60%
Hints
Which of the following compounds has zero dipole moment?
1.
2.
3.
4.
Subtopic: Polarity |
83%
Level 1: 80%+
Hints
The linear molecule among the following is
1.
2.
3.
4.
Subtopic: V.S.E.P.R & V.B.T |
91%
Level 1: 80%+
Hints
The species which has triangular planar geometry is
1.
2.
3.
4.
Subtopic: Hybridisation |
77%
Level 2: 60%+
Hints
The correct order of stability for the following species is:
| 1. | Li2 < He2+ < O2+ < C2 |
| 2. | C2 < O2+< Li2 < He2+ |
| 3. | He2+< Li2 < C2 < O2+ |
| 4. | O2+ < C2 < Li2 < He2+ |
Subtopic: M.O.T |
59%
Level 3: 35%-60%
Hints
From the given structures, the correct structure(s) of is are
l. 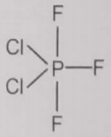
ll. 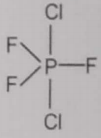
lll. 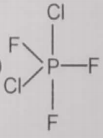
1. Only l
2. Only ll
3. Only lll
4. l, ll and lll
Subtopic: Hybridisation |
64%
Level 2: 60%+
Please attempt this question first.
Hints
The pair that is isostructural (i.e. having the same shape and hybridization) is
1.
2.
3.
4.
Subtopic: Hybridisation |
90%
Level 1: 80%+
Hints






