The linear molecule among the following is
1.
2.
3.
4.
The species which has triangular planar geometry is
1.
2.
3.
4.
The correct order of stability for the following species is:
| 1. | Li2 < He2+ < O2+ < C2 |
| 2. | C2 < O2+< Li2 < He2+ |
| 3. | He2+< Li2 < C2 < O2+ |
| 4. | O2+ < C2 < Li2 < He2+ |
From the given structures, the correct structure(s) of is are
l. 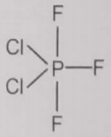
ll. 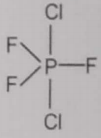
lll. 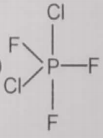
1. Only l
2. Only ll
3. Only lll
4. l, ll and lll
The pair that is isostructural (i.e. having the same shape and hybridization) is
1.
2.
3.
4.
Which of the following represents the correct order of dipole moment?
1.
2.
3.
4.
Which of the following has the shortest bond length?
1.
2.
3.
4.
Lattice energy for an ionic compound is calculated by using
1. Kirchhoff's equation
2. Markownikoff's rule
3. Born Haber cycle
4. Carnot cycle
Assuming that Hund's rule is violated by the diatomic molecule , its bond order and magnetic nature will be respectively
1. 1, diamagnetic
2. 1, paramagnetic
3. 2, diamagnetic
4. 2, paramagnetic






