The melting point of is and that of is (it sublimes) because:
1. There is a very large difference in the ionic character of the Al - F and Si - F bonds
2. In interacts very strongly with the neighboring ions to give a three-dimensional structure but in no such interaction is possible
3. The silicon ion in the tetrahedral is not shielded effectively from the fluoride ions whereas in ion is shielded on all sides
4. The attractive forces between the molecules are strong whereas those between the molecule are weak
In the series ethane, ethylene and acetylene, the C - H bond energy is
1. The same in all the three compounds
2. Greatest in ethane
3. Greatest in ethylene
4. Greatest in acetylene
A simplified application of MO theory to the hypothetical 'molecule' OF would give its bond order as
1. 2
2. 1.5
3. 1.0
4. 0.5
The bond order depends on the number of electrons in the bonding and antibonding orbitals.
Which of the following statements are correct about bond order?
1. Bond order can have a negative value.
2. It always has an integral value.
3. It is always a non zero quantity
4. It can assume any value-positive or fractional, including zero.
What is the best description of the geometry of the nitrogen atoms in dimethylnitrosamine, ?
N bounded to groups N bonded to O
1. Trigonal planar Linear
2. Trigonal planar Bent
3. Trigonal pyramidal Linear
4. Trigonal pyramidal Bent
The following diagram is sometimes used to illustrates the structure of benzene
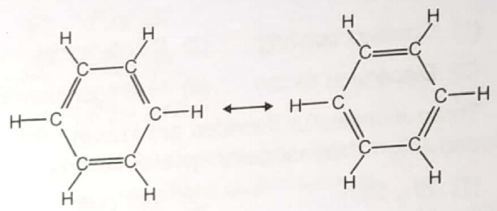
Which of the statements concerning the structure of benzene is false?
1. The double bonds oscillate rapidly back and forth between adjacent pairs of carbon atoms
2. The
3. The carbon atoms form a flat hexagonal ring
4. Above structures are resonance structures
Removing an electron from molecular oxygen, , to form the dioxygenyl cation, , causes what changes in the bond length and in the number of unpaired electrons?
Bond length Number of unpaired electrons
1. Increase Increase
2. Increase Decrease
3. Decrease Increase
4. Decrease Decrease
How many carbon-carbon double bonds are present in linolenic acid, ?
1. 1
2. 2
3. 3
4. 4
The formulae and boiling points of three compounds are given in this table.
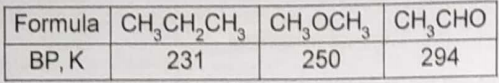
The trend in boiling points is best attributed to variations in
1. Covalent bonding
2. Dipole forces
3. Dispersion forces
4. Hydrogen bonding




