Removing an electron from molecular oxygen, , to form the dioxygenyl cation, , causes what changes in the bond length and in the number of unpaired electrons?
Bond length Number of unpaired electrons
1. Increase Increase
2. Increase Decrease
3. Decrease Increase
4. Decrease Decrease
How many carbon-carbon double bonds are present in linolenic acid, ?
1. 1
2. 2
3. 3
4. 4
The formulae and boiling points of three compounds are given in this table.
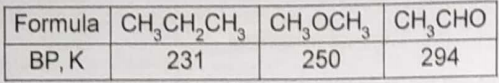
The trend in boiling points is best attributed to variations in
1. Covalent bonding
2. Dipole forces
3. Dispersion forces
4. Hydrogen bonding
Three monosulfur fluorides are known : . Of these, polar species include
1. Only
2. Only
3. and Only
4. , and
Which ionic compound has the largest lattice energy?
1. LiF
2. BeO
3. KBr
4. CaS
The boiling points of are , respectively. This increase is best attributed to an increase in which of the following?
l. Dipole-dipole interactions.
ll. Dispersion forces.
lll. Hydrogen bonding.
1. l only
2. I and ll
3. lll only
4. ll and lll only
The lattice energy (energy required to separate the ions in an ionic solid) of MgO is much larger than that of LiF.
What contributes the most to this difference?
1. is a smaller ion than and is a smaller ion than
2. F is more electronegative than O, and Li is more electropositive than Mg
3. MgO contains doubly charged ions, while LiF contains singly charged ions
4. MgO contains more electrons than LiF
Allene has the structure . What is the best description of the geometry of allene?
Geometry at central carbon Position of hydrogen atoms
1. Linear All in the same plane
2. Linear In two perpendicular planes
3. Bent All in the same plane
4. Bent In two perpendicular planes
Which property or properties of metals can be accounted for by the electron sea model?
l. Electrical conductivity
ll. Malleability
1. l only
2. ll only
3. Both l and ll
4. Neither l nor ll
What is the principal energetic factor in the lack of miscibility between and ?
1. The strength of intermolecular forces of attraction between molecules
2. The strength of intermolecular forces of attraction between molecules
3. The differences between the molecular weights of the molecules
4. The difference in electronegativity between carbon and hydrogen




