Which of the following carbocations is the most stable?
1.

2.
\(\mathrm{(CH}_3){ }_3 \stackrel{\oplus}{\mathrm{C}}\)
3.
\(\mathrm{CH}_2=\mathrm{CH}-\stackrel{\oplus}{\mathrm{C}\mathrm{H}_2}\)
4.
\(\left(\mathrm{CH}_3\right)_2 \stackrel{\oplus}{\mathrm{C H}}\)

How many chiral centers are present in 3,4-dibromo-2-pentanol?
1. 1
2. 2
3. 3
4. 4
Which of the following compounds is an isomer of methyl vinyl ether?
1. Propanal
2. 1-Propanol
3. Ethyl methyl ether
4. Dimethylether
The molecule among the following having the highest reactivity toward the SN1 reaction is:
1. C6H5CH(C6H5)Br
2. C6H5CH(CH3)Br
3. C6H5C(CH3)(C6H5)Br
4. C6H5CH2Br
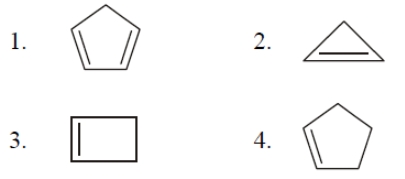
The aromatic compound among the following is :
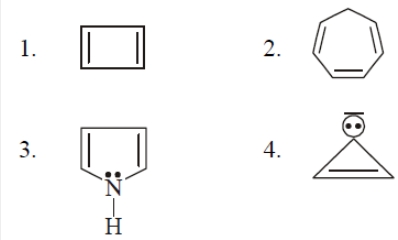
Which alkene is most stable ?
1.
2.
3.
4.
The correct order of the rate of expulsion of hydrogen as in the above figure is:
1. 1 > 2 > 3
2. 2 > 3 > 1
3. 2 > 1 > 3
4. 3 > 2 > 1
Consider the following resonating structures of HCOOH
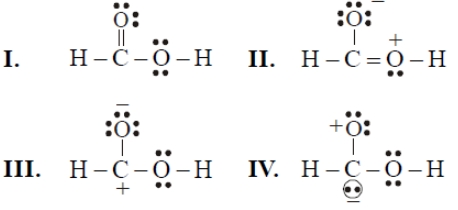
The order of stability is-
| 1. | I>II>III>IV | 2. | IV>I>II>III |
| 3. | I>III>II>IV | 4. | II>I>III>IV |
The correct order of abstraction of hydrogen toward homolytic fission is:
1. 2 > 4 > 3 > 5 > 1
2. 4 > 2 > 5 > 3 > 1
3. 4 > 2 > 3 > 5 > 1
4. 4 > 3 > 2 > 5 > 1