Photons of wavelength 4000 are used to break molecules. The percentage of energy converted to the kinetic energy of atoms will be :
(bond dissociation energy of the molecule is 246.5 kJ/mol)
1. 12%
2. 8%
3. 26%
4. 17%
A photon with an initial frequency of \(10^{11}~\mathrm{Hz}\) scatters off an electron at rest. Its final frequency is \(0.9 \times10^{11}~\mathrm{Hz}.\) The speed of scattered electron is close to:
| 1. | \(3 \times10^{2}~\mathrm{ms}^{-1}\) | 2. | \(3.8 \times10^{3}~\mathrm{ms}^{-1}\) |
| 3. | \(2 \times10^{6}~\mathrm{ms}^{-1}\) | 4. | \(30~\mathrm{ms}^{-1}\) |
The ratio of the area of the orbit of the first excited state of an electron to the area of the orbit of ground level for hydrogen atom will be
1. 4:1
2. 8:1
3. 16:1
4. 2:1
Of the following transitions in hydrogen atom, the one which gives an absorption line of lowest frequency is:
1. n = 1 to n = 2
2. n = 3 to n = 8
3. n = 2 to n = 1
4. n = 8 to n = 3
The total number of electron present in Cu Which have zero value of magnetic quantum number in the ground state
1. 13
2. 7
3. 8
4. 15
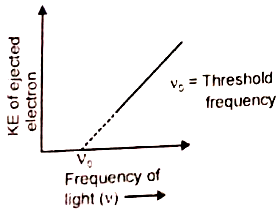
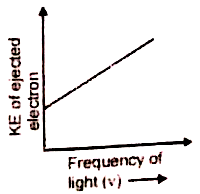
Which of the given graphs depicts the correct relation between the kinetic energy of photoelectrons and the frequency of incident light (v)?
1. 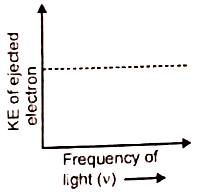 2.
2. 
3. 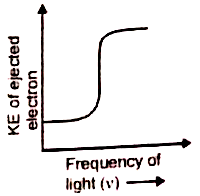 4.
4. 
The energy of a photon of wavelength l = 1 meter is (Planck's constant = 6.626 Js, speed of light )
1. 1.988 x
2. 1.988 x
3. 1.988 x
4. 1.988 x
For a one-electron atom, the set of allowed quantum numbers is
1. n = 1, l = 0, = 0, = +1/2
2. n = 1, l = 1, = 0, = +1/2
3. n = 1, l = 0, = -1, = -1/2
4. n = 1, l = 0, = 1, = -1/2
A metal is irradiated with light of wavelength 660 nm. Given that the work function of the metal is I .0 eV. the de-Broglie wavelength of the ejected electron is close to
1. 6.6 x m
2. 1.3 x m
3. 8.9 x m
4. 6.6 x m
The energy of an electron in the first Bohr orbit is
1. -30.6 eV
2. -40.8 eV
3. -54.4 eV
4. +40.8 eV






