The activation energies of two reactions are and with > .
f the temperature of the reacting system is increased from T1 to T2 .
The correct relation is:
1.
2.
3.
4.
f the temperature of the reacting system is increased from T1 to T2 .
The correct relation is:
The units of rate constant and rate of reaction are same for :
1. First order reaction
2. Second order reaction
3. Third order reaction
4. Zero order reaction
The plot of log k vs helps to calculate :
1. Energy of activation.
2. Rate constant of reaction.
3. Order of the reaction.
4. Energy of activation as well as the frequency factor.
Rate constant K = 1.2×103 mol–1 L s–1 and Ea = 2.0×102 kJ mol–1. When T , A is equal
to
1. 2.0 × 102 kJ mol–1
2. 1.2 × 103 mol–1L s–1
3. 1.2 × 103 mol L–1 s–1
4. 2.4 × 103 kJ mol–1L s–1
The rate constant for a reaction 2×10–2 s–1 at 300 K and 8×10–2 s–1 at 340 K. The energy of activation of the reaction is:
1. 14.69 kJ mol–1
2. 29.39 kJ mol–1
3. 44.34 kJ mol–1
4. 22.05 kJ mol–1
The reaction 2A + B + C D + E is found to be a first-order reaction with respect to A, second-order reaction with respect to B, and zero-order reaction with respect to C. If the concentrations of A, B, and C are doubled, the rate of the reaction will be:
1. 72 times
2. 8 times
3. 24 times
4. 36 times
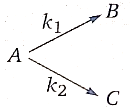
1. 757.48 K
2. 378.74 K
3. 600.91 K
4. None of the above
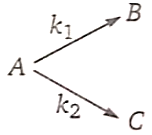
1. 100 kJ/mol
2. 120 kJ/mol
3. 116 kJ/mol
4. 220 kJ/mol






