This video is available for NEETprep Video Course students only
You’ve reached the end of your free Videos limit.
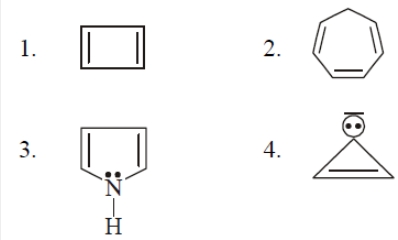
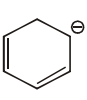
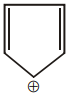
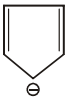
#61 | Aromaticity
(Chemistry) > Organic Chemistry - Some Basic Principles And Techniques
My Notes
Create your notes while watching video by clicking on icon in video player.
Related Practice Questions :