Doubt by Divya devangada
It can be dolike this . then answer is different.this method right or wrong
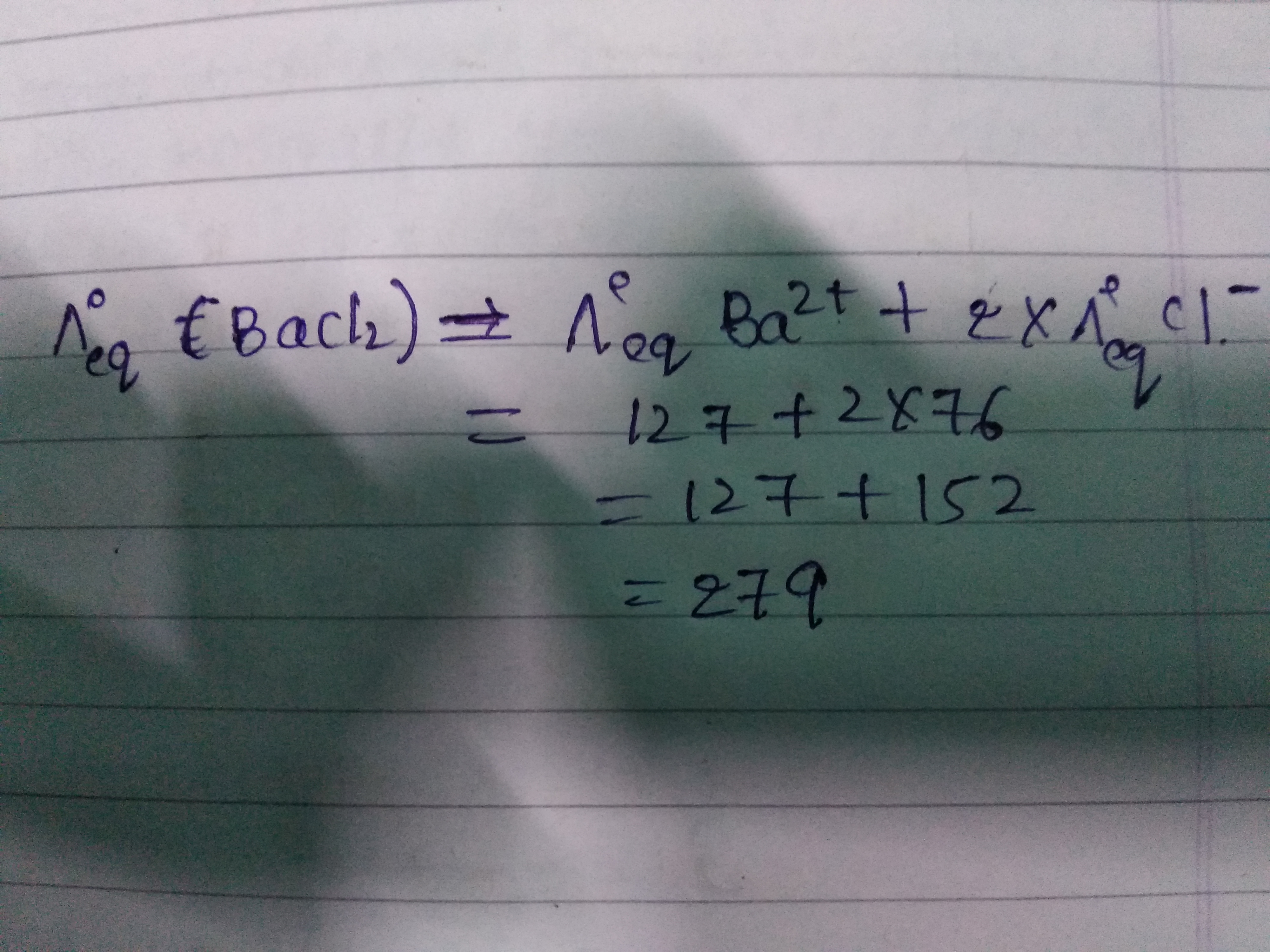
Answers
Answer by nitesh chaudhary
If you read the question carefully, the equivalent conductivity of Ba2+BaX2+ and Cl−1ClX−1 are provided to you.
So the molar conductivity of Ba2+BaX2+ is: 2×127 ohm−1mole−1=254
and that of Cl−1=76
Now apply Kohlrausch's law of molar conductivity of solution at infinite dilution:
𝜆𝑜BaCl2=𝜆𝑜Ba2++2𝜆𝑜Cl−1⟹𝜆𝑜BaCl2=254+2×76=406 Now, , equivalent conductivity is 1/2 times the molar conductivity for BaCl2BaClX2 so equivalent conductivity of BaCl2=1/2×406=203
