Doubt by Reji
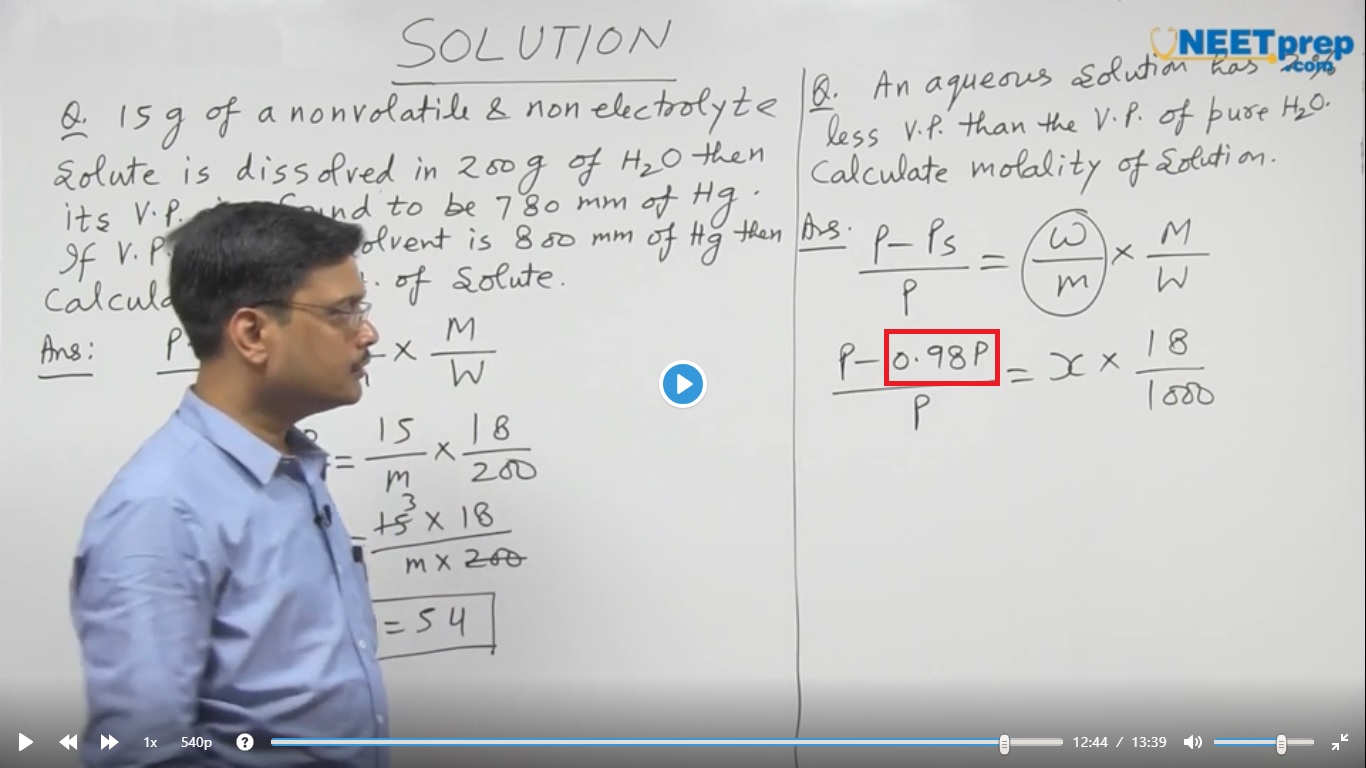
sir..here how does the value of Ps 0.98P came???
Answers
Answer by Rishikant Pandey
moles of benzene and toluene both are same means equal amount of both are mixed
hence we know that mole fraction is number of moles divided by total number of moles of solution
mole fraction (Xb) for benzene Nb/Nb+Nt
molefraction (Xt )for toluene Nt/Nb+Nt
Nb----no. of moles of benzene
Nt----no. of moles of toluene
given:
Nb=Nt =>Xb=Xt-------(1)
Xt+Xb=1 --------(2)
from 1&2
2Xt=1
Xt=1/2=0.5
Xt=Xb=0.5
Answer by Rishikant Pandey
i apologize for answer its by mistake given to you
Answer by Rishikant Pandey
correct answer is
pure water vapour pressure is 100% in numeric it is 1
but here given vapour pressure is 2% in numeric fraction it is 0.02
1-0.02=0.98
