Doubt by Divyanshi sahu
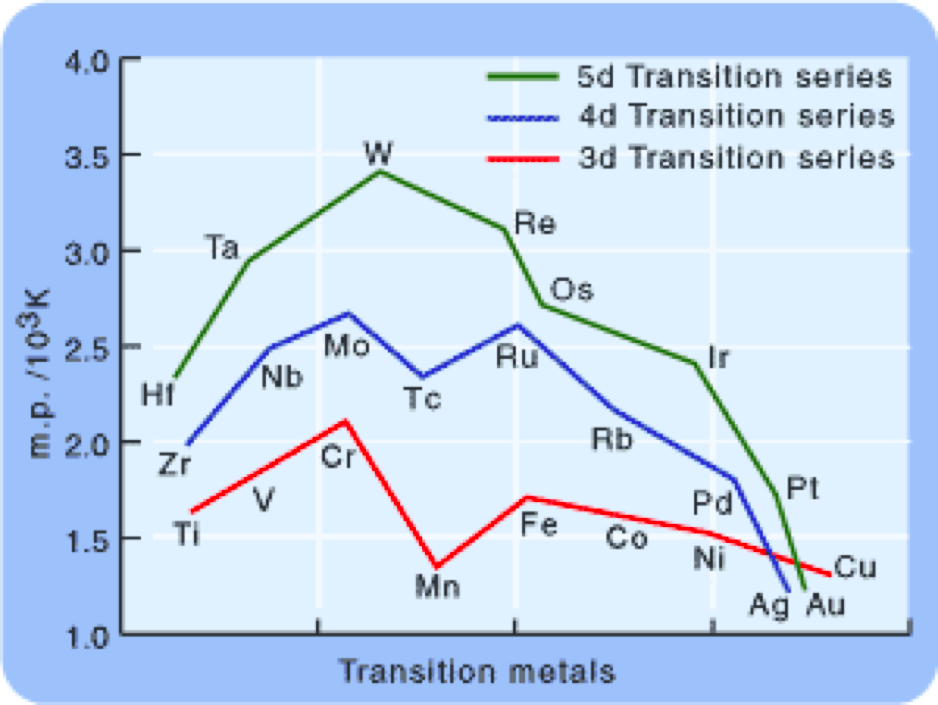
As we know that melting and boiling point of D block elements are higher due to presence of unpaired electrons but we know that z-effective exceptionally increases in D block. and z-effective increases then metallic bond strength increases and it is inversely proportional to melting point so melting point should decrease??
Answers
Answer by Dr. Rakhee
We can't say that melting point of any d-block element is totally depend on metallic character or size of atom or Zeff value. Since it is experimental value totally depend on type of crystalline structure of any particular d-block element.
I send you the melting point for d-block elements
see it and focus what I told to you.
Answer by Divyanshi sahu
Mam melting point of chromium is greater than melting point of vanadium...why its value is shown lesser??
Answer by Divyanshi sahu
Thanks mam for the explanation