Doubt by sonu kumar
please explain which is the correct order of acidic strength
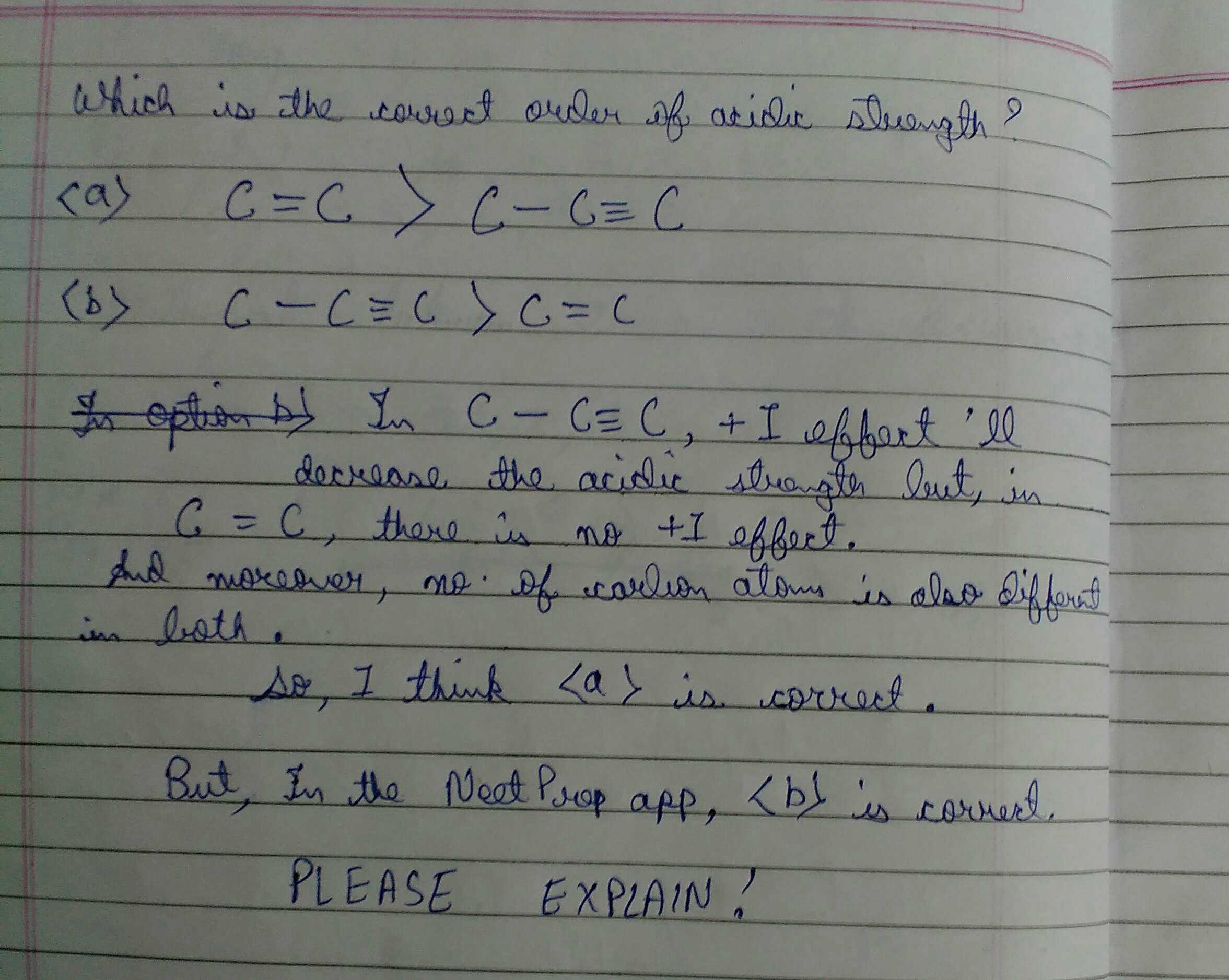
Answers
Answer by nitesh chaudhary
Carbon atoms in alkynes are sp hybridized
Alkenes sp2
Alkanes sp3
Hence %s character -50,33,25 resp.
An electron in p orbital is at a larger distance from the nucleus and is relatively loosely held,whereas an electron in s orbital is closer to the nucleus and is held more tightly.
Thus in hybridized state,electronegativity of carbon increases with increase in s character.
Hence alkynes can loose a proton relatively easily.
+I effect comes in picture when u r comparing two alkynes.
